
Welcome to another edition of “Ophthalmology 360.” This column is put together by the members of CEDARS (Cornea, External Disease, and Refractive Surgery Society), ASPENS (American Society of Progressive Enterprising Surgeons), and Vanguard Ophthalmology.
As IOL technology continues to improve, patients’ demands and expectations grow as well. The quest for the perfect lens, which allows both excellent distance and near vision without correction, has become the holy grail of cataract surgery. Unfortunately, despite their outstanding attributes, the lenses currently available still have limitations. In this edition, Cynthia Matossian, MD, discusses some new lenses on the horizon. I hope you enjoy this article.
—Kenneth A. Beckman, MD, section editor

Extended-depth-of-focus (EDOF) IOLs and extended-range-of-vision (ERV) IOLs are implants that use entirely new design concepts to provide an extended range of crisp vision and a high rate of spectacle independence. These lenses are associated with a low incidence of photic phenomena such as halos and glare, according to the manufacturers.
MINIMAL FOCUS ON RESBYOPIA-CORRECTING IMPLANTS
Even though more than 3.6 million cataract surgery procedures are performed annually in the United States, according to Market Scope’s 2014 survey, only 4.6% involve presbyopia-correcting implants. Whether multifocal or accommodating, both options have their limitations.
Consequently, many ophthalmologists shy away from these choices and offer mini-monovision, in which the dominant eye is usually targeted for distance and the nondominant eye for the intermediate/near range, with a planned dioptric IOL power difference between eyes.
EXTENDING THE RANGE OF VISION, DEPTH OF FOCUS
The Tecnis Symfony Extended Range of Vision IOL (Abbott Medical Optics), available in toric and nontoric versions, is indicated to provide a full range of continuous, high-quality vision without some of the limitations of multifocal IOLs. Although this new ERV implant (Figure 1) looks like a diffractive multifocal optic, its unique design is intended to deliver well-focused vision over an enhanced range, along with 50% less light loss than with a traditional diffractive implant model. The lens material also corrects for chromatic aberration (Figure 2). Most chromatic aberration arises from the dispersion of light through the optic material. Increasing the Abbe number of the optic material improves the overall optical performance of the implant (Figure 3). The defocus curve established in clinical trials thus far shows ample uncorrected intermediate vision in addition to clear distance and functional near visual acuities (data on file with Abbott Medical Optics).
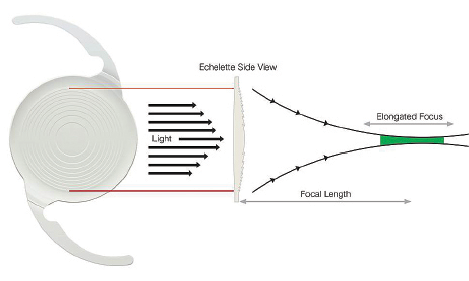
Figure 1. Schematic of the Tecnis Symfony Extended Range of Vision IOL.
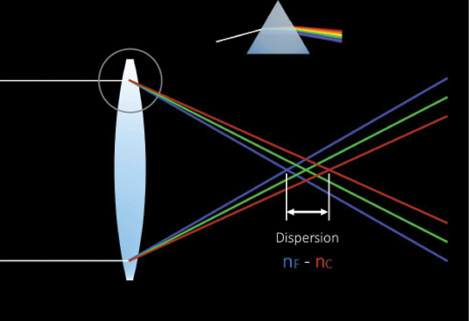
Figure 2. Chromatic aberration.
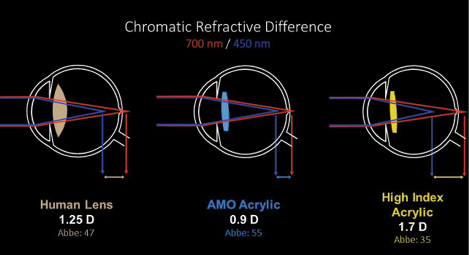
Figure 3. Chromatic refractive difference.
This IOL became available in Europe last year. Enrollment has closed in an FDA study of the IOL, and the results are expected to be monitored during 2015.
Hoya Surgical Optics is developing EDOF implants, but they are not yet available in the United States.
The Softec HD IOL from Lenstec has some properties that make it similar to EDOF lenses. Because the Softec HD IOL is made of a hydrophilic acrylic material, it is more pliable than hydrophobic acrylic IOLs and may provide some intermediate vision, according to the company and my own retrospective data. Moreover, coupling the zero spherical aberration of this implant with the cornea’s natural positive spherical aberration may give an overall positive spherical aberration for EDOF with functional intermediate vision.
PREMIUM OR NEW TECHNOLOGY DESIGNATION POSSIBLE
According to Donna McCune, vice president of Corcoran Consulting Group, EDOF and ERV IOLs may fall into a new class of premium-channel or new-technology IOLs (NTIOLs), as designated by the FDA.
Currently, only presbyopia- and astigmatism-correcting IOLs are designated as premium implants by the Centers for Medicare & Medicaid Services, but there are no active NTIOLs, because the NTIOL status of previous products has expired.
If EDOF or ERV IOLs receive NTIOL designations, then an out-of-pocket charge to patients may be possible. Additional information on the process may be found at www.cms.gov/medicare/medicare-fee-for-service-payment/ascpayment/ntiols.html.
Although no one can say with certainty what may happen with this new category of lenses, EDOF or ERV IOLs may become available in the United States in the not-too-distant future. n
Kenneth A. Beckman, MD
• clinical assistant professor of ophthalmology at Comprehensive EyeCare of Central Ohio, Ohio State University, Westerville, Ohio
• (614) 890-5692; kenbeckman22@aol.com; Twitter @kab822
Cynthia Matossian, MD
• founder and CEO of Matossian Eye Associates, with offices in Pennsylvania and New Jersey
• clinical instructor/adjunct faculty member at Temple University School of Medicine in Philadelphia and Robert Wood Johnson Medical School in New Brunswick, New Jersey
• cmatossian@matossianeye.com
• financial disclosure: speaker for and consultant to Abbott Medical Optics, speaker for LensTec


