Dispelling Misconceptions About Calcification Risks and Performance
By Abinaya Thenappan, MD and Shamik Bafna, MD


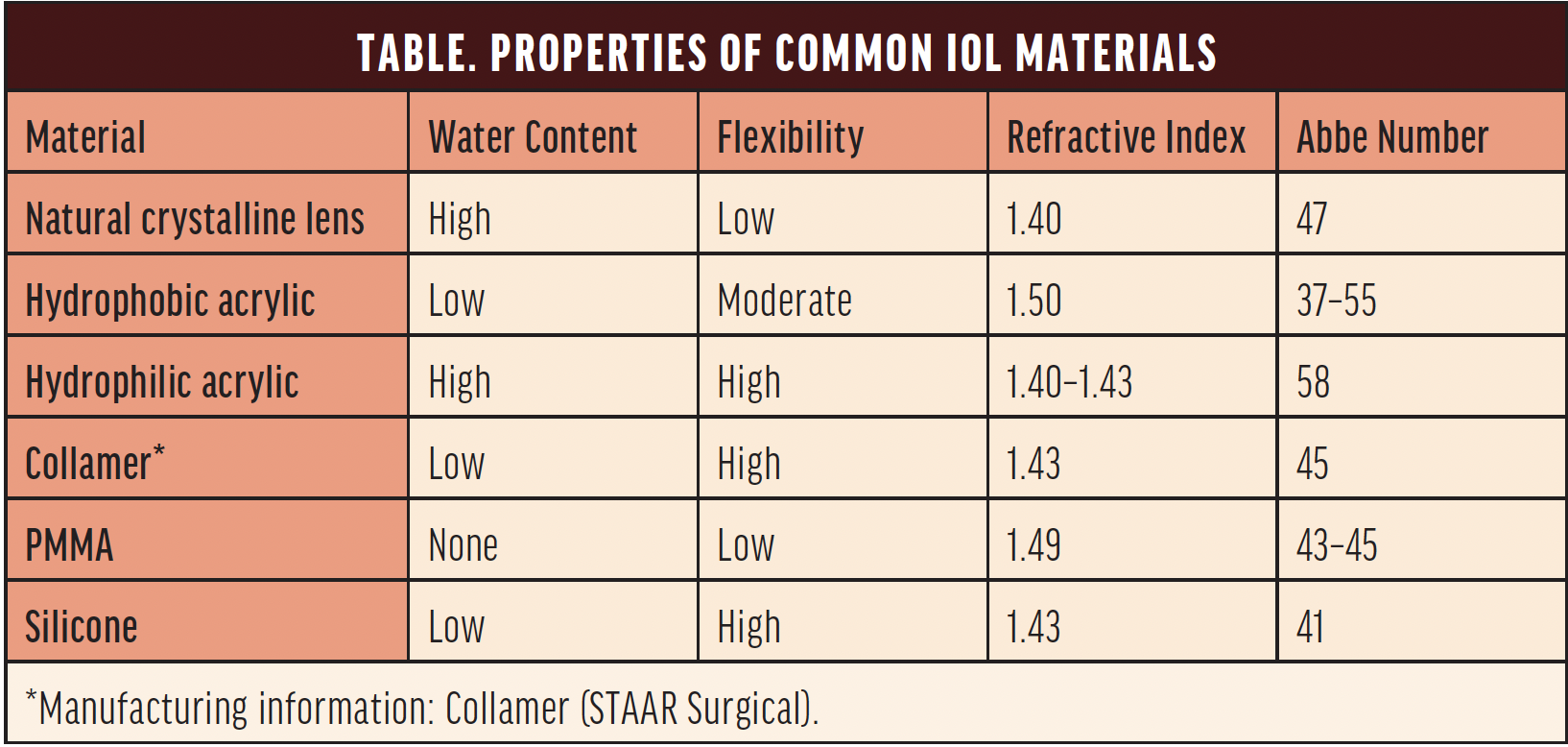
A wide range of IOL materials is available, each with unique physical and optical properties (Table). Hydrophilic acrylic lenses were initially favored for their low refractive index, but early reports of calcification and opacification raised concerns about their long-term performance.1-4 In a 2001 survey, hydrophilic acrylic IOLs accounted for 28% of all explantations, with 98% of these attributed to calcification.5

Since their introduction in 1993, hydrophobic acrylic IOLs such as the AcrySof (Alcon) have dominated the market largely owing to a reduced risk of posterior capsular opacification (PCO) formation and a lower incidence of calcification. Hydrophilic acrylic IOLs nevertheless continue to hold a significant share of the market and account for approximately 29% of the IOLs implanted worldwide.6 Advances in technology and design have addressed many earlier concerns, leading to renewed interest in and scrutiny of hydrophilic IOLs.
This article attempts to debunk prevalent myths about hydrophilic acrylic IOLs and explores their risks and benefits to foster a comprehensive understanding of their role in contemporary cataract surgery.
HYDROPHILIC VERSUS HYDROPHOBIC ACRYLIC IOLS
The hydrophilicity or hydrophobicity of an IOL material is determined by measuring the angle formed when a water droplet is placed on the material’s surface. Hydrophobic materials have a large contact angle, whereas hydrophilic materials have a small contact angle.
The water content of hydrophilic and hydrophobic IOLs is 18% to 38% and 0.5% to 1%, respectively.7 A higher water content gives hydrophilic acrylic IOLs greater biocompatibility, flexibility, and ease of handling during implantation compared to hydrophobic acrylic IOLs.
THE RISK OF CALCIFICATION WITH MODERN HYDROPHILIC ACRYLIC IOLS
Concerns about the opacification of hydrophilic acrylic IOLs date back to early reports of calcification in patients undergoing procedures that required an intraocular injection of air or gas, such as endothelial keratoplasty, glaucoma surgery, and pars plana vitrectomy.1-4,8 The first generations of hydrophilic acrylic IOLs were associated with internal or surface calcification due to the deposition of calcium and phosphate.9 Because it was impossible to predict which patients might need such procedures in the future, it was suggested that cataract surgeons avoid hydrophilic acrylic IOLs entirely.2 Concern over calcification was heightened for two reasons: (1) IOL explantation is the only effective treatment for visually significant opacification and (2) a secondary IOL is often required because it may not be feasible to preserve the capsular bag.
Technological and manufacturing advances have addressed many early concerns regarding hydrophilic acrylic lenses. For instance, modern surface treatments, anticalcification coatings, and enhanced optic designs have been developed to improve the longevity and performance of hydrophilic acrylic lenses. Additionally, a growing body of evidence challenges the notion that hydrophilic acrylic lenses are inherently prone to opacification. A retrospective study published in 2020 found that patients with healthy eyes and those with comorbidities such as macular degeneration, glaucoma, proliferative diabetic retinopathy, and amblyopia achieved excellent visual outcomes with the CT Asphina model 409 (Carl Zeiss Meditec), a monofocal aspheric hydrophilic acrylic lens.10 The rate of Nd:YAG laser capsulotomy was 9.9% among these eyes, and there was no reported calcification in the entire cohort of more than 200 eyes during a 12-month follow-up period.10 Another study published in 2024 examined all lenses explanted within 1 year after surgery and found that only 16% (eight out of 50) were explanted owing to the opacification of a hydrophilic IOL or a hydrophilic IOL with a hydrophobic surface.11 Although this study reported on the performance of hydrophilic acrylic lenses over a 1-year period, it is important to note that most lens calcification is observed 2 to 10 years after surgery.
BENEFITS OF MODERN HYDROPHILIC ACRYLIC IOLS
Visual Outcomes
Modern hydrophilic acrylic IOLs can provide excellent visual outcomes. In some studies, these lenses have performed on par with or better than hydrophobic acrylic IOLs.12 A notable advantage of hydrophilic IOLs is their resistance to glistenings and inclusions commonly found in older hydrophobic acrylic IOLs.13,14 Glistenings are fluid-filled microvacuoles within the polymer of the lens optic. They are believed to scatter light and negatively affect patients’ quality of vision, although the magnitude of this effect on their postoperative visual function remains uncertain.
The zero-aberration aspheric design of many hydrophilic acrylic IOLs optimizes visual performance, potentially providing patients with clearer vision. Traditional biconvex IOLs with spherical surfaces often have positive spherical aberration, which compounds the cornea’s natural positive spherical aberration. In contrast, aspheric IOLs are designed to add negative spherical aberration, thus aligning more closely with the eye’s natural aberration profile.
Finally, hydrophilic IOLs tend to have a higher Abbe number than hydrophobic IOLs (Table), indicating improved overall visual clarity. The Abbe number of a lens, which ranges from 35 to 60 for modern IOLs,15 reflects the degree of light dispersion within the lens. A higher Abbe number corresponds to less chromatic aberration, reduced light dispersion, and improved color correction.
Biocompatibility
The high water content of hydrophilic acrylic IOLs makes them more compatible with the eye’s natural tissues and results in a lower rate of glare and a lower refractive index. The likelihood of inflammatory responses in patients with comorbidities such as uveitis and diabetes is low because the high biocompatibility of these lenses leads to quiet eyes and excellent visual outcomes.16,17
RISKS ASSOCIATED WITH HYDROPHILIC ACRYLIC IOLS
Hydrophilic acrylic IOLs carry a higher risk of PCO than other types of IOLs. The hydrophilic lens surface can promote the growth of lens epithelial cells on the posterior capsule, leading to increased opacification. This contrasts with hydrophobic IOLs, which adhere to the posterior lens capsule and limit the space for lens epithelial cell migration between the IOL and the posterior capsule.18,19 Two meta-analyses comparing PCO prevalence found that the rate of PCO was lower with hydrophobic than with hydrophilic IOLs, although this difference was not associated with superior visual acuity.18,19
Design factors may contribute to the incidence of PCO. The introduction of lens optics with square edges has reduced the incidence of PCO in both hydrophobic and hydrophilic lenses. Studies have suggested that the design of square edges varies, and they are less pronounced in hydrophilic IOLs compared to hydrophobic IOLs,20 which may contribute to differences in PCO rates.
Patient-related factors such as age, preexisting ocular conditions, and surgical complexity can also influence PCO formation. For instance, individuals who have a history of anterior segment pathology or those undergoing complex surgical procedures may be at increased risk of PCO with hydrophilic IOLs. Careful consideration of these variables can help surgeons select the most appropriate IOL type to optimize patient outcomes and reduce the incidence of PCO.
WHEN TO CHOOSE A HYDROPHILIC ACRYLIC IOL
No single IOL can address every possible clinical scenario or patient need because each has its advantages and limitations. The unique characteristics of hydrophilic acrylic IOLs make them an excellent choice for three specific patient groups.
▶ No. 1: Patients With High Myopia or Astigmatism
The design of hydrophilic acrylic IOLs minimizes optical aberrations, which is important for achieving good visual outcomes in such cases.
▶ No. 2: Patients Who Have a History of Uveitis or Other Inflammatory Conditions
Compared to other lens materials, hydrophilic IOLs are less likely to cause inflammatory reactions. This makes them a preferred choice for patients with a history of uveitis or other inflammatory conditions because the high biocompatibility of these lenses reduces the risk of postoperative inflammation.
▶ No. 3: Patients With Limited Access to Advanced Surgical Facilities
In areas where patient access to advanced surgical facilities and resources is limited, the cost-effectiveness of hydrophilic IOLs becomes important. Additionally, the relatively straightforward handling characteristics and stable performance of these lenses make them a reliable choice in such settings.
A Posterior Perspective
By Harrison Sciulli, MD and Llewelyn Rao, MD


Hydrophilic acrylate has been used in IOLs for at least 40 years and became a common lens material in the 1990s. Since then, posterior segment surgeons have been tasked with finding solutions for complicated scenarios such as the removal of dislocated IOLs.21 When explanting an IOL, surgeons must decide which replacement lens to use and how to implant it.
This article discusses the scleral fixation of hydrophilic acrylic IOLs. Good refractive outcomes have been reported with several of these lenses. In a recent study, “the mean best-available logMAR visual acuity improved from 1.61 (~20/800) preoperatively (0.025 decimal equivalent) to 0.57 (~20/80) postoperatively (0.3 decimal equivalent), this difference being statistically significant (P < 0.001).”22
THE ADVANTAGES OF HYDROPHILIC ACRYLIC LENSES FOR SUTURE FIXATION
There are advantages to selecting a hydrophilic acrylic IOL for scleral fixation after the removal of a dislocated lens. First, hydrophilic acrylic lenses are flexible enough to be folded through a small corneal incision, which can limit surgically induced astigmatism and result in a more controlled refractive outcome. Second, the refractive indices of these lenses tend to be high,23 allowing the IOLs to be very thin. When the IOLs are placed at the appropriate distance, iris chafing is minimal, and the risk of uveitis-glaucoma-hyphema syndrome is low.
The Akreos AO60 lens (Bausch + Lomb) is particularly well suited for this procedure. Its four closed-loop haptics allow for four points of scleral fixation,24 creating a highly stable lens complex when a PTFE suture is used. An informal survey of the physicians at Retina Associates of Cleveland, where we practice, found that none of us has seen one of these lenses become dislocated since we began performing the procedure in 2016. It is also worth noting that these lenses offer a pristine view of the posterior segment.
RISKS
Opacification
Most complex lens procedures performed by posterior segment surgeons involve a vitrectomy to remove remnants of the natural lens and the dislocated IOL. Although these maneuvers are generally safe, there is always a risk of postoperative retinal detachment, especially after complicated cataract surgery. When a retinal detachment is repaired with a vitrectomy, an air-fluid exchange is performed once the retina is flat and the breaks have been treated. During this procedure, inert gas or oil is injected into the eye to allow the retina time to form scars around the breaks. This fluid may remain in place for months.
A risk of opacification has been reported when an air-gas exchange is performed or silicone oil is instilled into eyes with hydrophilic acrylic IOLs.25-28 Our group has performed scleral fixation of a total of 249 Akreos lenses since 2016. An informal survey found that two physicians in our group have encountered opacification of an Akreos lens following air-gas exchanges. In only one of the two cases was the opacification visually significant enough to necessitate an IOL exchange. Suture fixation of a new Akreos lens was performed, and no further opacification has occurred.
Other Complications
Additional risks of the scleral fixation of a hydrophilic acrylic IOL include the development of proliferative vitreoretinopathy membranes on the lens, distortion of the lens optic due to tight suturing of the IOL to the sclera, cystoid macular edema, hypotony from a wound leak, vitreous hemorrhage, suture exposure necessitating a return to the OR for conjunctiva repair, and reverse pupillary block syndrome. All of these complications are inherent to sutured lens procedures with IOLs composed of any material.
CONCLUSION
Some surgeons are hesitant to perform scleral fixation of a hydrophilic acrylic IOL because of the risk of opacification. Although this is a potential complication, the reliability, stability, and clarity of these lenses make them our preferred choice for the technique.
1. Marcovich AL, Tandogan T, Bareket M, et al. Opacification of hydrophilic intraocular lenses associated with vitrectomy and injection of intraocular gas. BMJ Open Ophthalmol. 2018;3(1):e000157.
2. Grzybowski A, Zemaitiene R, Markeviciute A, Tuuminen R. Should we abandon hydrophilic intraocular lenses? Am J Ophthalmol. 2022;237:139-145.
3. Fellman MA, Werner L, Liu ET, et al. Calcification of a hydrophilic acrylic intraocular lens after Descemet-stripping endothelial keratoplasty: case report and laboratory analyses. J Cataract Refract Surg. 2013;39(5):799-803.
4. Giers BC, Tandogan T, Auffarth GU, et al. Hydrophilic intraocular lens opacification after posterior lamellar keratoplasty – a material analysis with special reference to optical quality assessment. BMC Ophthalmol. 2017;17(1):150.
5. Mamalis N. Complications of foldable intraocular lenses requiring explantation or secondary intervention–2001 survey update. J Cataract Refract Surg. 2002;28(12):2193-2201.
6. 2018 IOL Report: A Global Market Analysis for 2017 to 2023.
7. Werner L. Intraocular lenses: overview of designs, materials, and pathophysiologic features. Ophthalmology. 2021;128(11):e74-e93.
8. Memmi B, Knoeri J, Bouheraoua N, Borderie V. Intraocular lens calcification after pseudophakic endothelial keratoplasty. Am J Ophthalmol. 2023;246:86-95.
9. Neuhann IM, Werner L, Izak AM, et al. Late postoperative opacification of a hydrophilic acrylic (hydrogel) intraocular lens: a clinicopathological analysis of 106 explants. Ophthalmology. 2004;111(11):2094-2101.
10. Johansson B, Daniel ACS, Herbers C, Gerl M, Kretz FTA. Clinical safety and efficacy of a hydrophilic acrylic intraocular lens in a real-world population: a 1-year follow-up retro-prospective study. BMC Ophthalmol. 2020;20(1):224.
11. Friedrich M, Son HS, Hassel O, et al. Early intraocular lens explantations: 10-year database analysis. BMC Ophthalmol. 2024;24(1):300.
12. Bellucci C, Mora P, Tedesco SA, Gandolfi S, Bellucci R. Refractive outcome and 5-year capsulotomy rate of hydrophobic and hydrophilic IOLs with similar optical design: a contralateral study. Ophthalmol Ther. 2023;12(2):1387-1395.
13. Colin J, Orignac I, Touboul D. Glistenings in a large series of hydrophobic acrylic intraocular lenses. J Cataract Refract Surg. 2009;35(12):2121-2126.
14. Chang A, Kugelberg M. Glistenings 9 years after phacoemulsification in hydrophobic and hydrophilic acrylic intraocular lenses. J Cataract Refract Surg. 2015;41(6):1199-1204.
15. Zhao H, Mainster MA. The effect of chromatic dispersion on pseudophakic optical performance. Br J Ophthalmol. 2007;91(9):1225-1229.
16. Abela-Formanek C, Amon M, Schauersberger J, Kruger A, Nepp J, Schild G. Results of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in uveitic eyes with cataract: comparison to a control group. J Cataract Refract Surg. 2002;28(7):1141-1152.
17. Abela-Formanek C, Amon M, Kahraman G, Schauersberger J, Dunavoelgyi R. Biocompatibility of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in eyes with uveitis having cataract surgery: long-term follow-up. J Cataract Refract Surg. 2011;37(1):104-112.
18. Li Y, Wang J, Chen Z, Tang X. Effect of hydrophobic acrylic versus hydrophilic acrylic intraocular lens on posterior capsule opacification: meta-analysis. PLoS One. 2013;8(11):e77864.
19. Zhao Y, Yang K, Li J, Huang Y, Zhu S. Comparison of hydrophobic and hydrophilic intraocular lens in preventing posterior capsule opacification after cataract surgery: an updated meta-analysis. Medicine (Baltimore). 2017;96(44):e8301.
20. Werner L, Müller M, Tetz M. Evaluating and defining the sharpness of intraocular lenses: microedge structure of commercially available square-edged hydrophobic lenses. J Cataract Refract Surg. 2008;34(2):310-317.
21. Shahid SM, Flores-Sánchez BC, Chan EW, et al. Scleral-fixated intraocular lens implants-evolution of surgical techniques and future developments. Eye (Lond). 2021;35(11):2930-2961.
22. Leuzinger-Dias M, Lima-Fontes M, Rodrigues R, et al. Scleral fixation of Akreos AO60 intraocular lens using Gore-Tex Suture: an eye on visual outcomes and postoperative complications. J Ophthalmol. 2021;2021:9349323.
23. Tetz M, Jorgensen MR. New hydrophobic IOL materials and understanding the science of glistenings. Curr Eye Res. 2015;40(10):969-981.
24. Akreos. Bausch + Lomb. Accessed September 4, 2024. https://www.bauschsurgical.com/cataract/akreos/#TECHNICAL-DATA
25. Padidam S, Rougraff N, Melamud A, Levinson JD. Incidence of Akreos AO60 intraocular lens opacification after concurrent or subsequent intraocular oil or gas use. Retin Cases Brief Rep. Published online April 20, 2023. doi:10.1097/ICB.0000000000001434
26. Patel NA, Fan KC, Yannuzzi NA, et al. Akreos AO60 intraocular lens opacification after retinal detachment repair. Ophthalmol Retina. 2020;4(8):854-856.
27. Gregori NZ, Echegaray JJ, Flynn HW Jr. Opacification of Akreos hydrophilic acrylic lens after retinal detachment repair with silicone oil tamponade: a case report. Ophthalmol Ther. 2019;8(2):341-345.
28. Werner L, Wilbanks G, Nieuwendaal CP, et al. Localized opacification of hydrophilic acrylic intraocular lenses after procedures using intracameral injection of air or gas. J Cataract Refract Surg. 2015;41(1):199-207.




