
Routine endophthalmitis prophylaxis with an intracameral (IC) antibiotic for cataract surgery is vastly underused in the United States compared with internationally. Since the ESCRS study, in 2007,1 and its discontinuation on ethical grounds, owing to the startling decrease in endophthalmitis in the treatment arm, many countries have made IC antibiotics available, and some have even made them mandatory. At the end of that landmark year, I began following the lead of Steve A. Arshinoff, MD, FRCSC, regarding the off-label use of moxifloxacin, and I never again saw endophthalmitis after cataract surgery in thousands of patients. The suggested moxifloxacin dosage has been adjusted upward to a minimum inhibitory concentration to overcome laboratory-measured resistance of organisms most likely implicated in endophthalmitis.
First, do no harm. There is ample evidence of the safety of IC moxifloxacin; I conducted one of many studies supporting this assertion.2 Significant risks have come to light for other antibiotic choices but not for self-preserved »Vigamox (moxifloxacin HCl ophthalmic solution 0.5%, Alcon), despite the manufacturer’s legal labeling added to the bottle that reads, “not for intraocular use.”
In addition to the enlightening meta-analysis by Bowen and colleagues that is the focus of this article,3 I would like to draw readers’ attention to an editorial published in Ophthalmology in 2016 that detailed a possible way to surmount the main barrier to IC prophylaxis in the United States: the lack of an FDA-approved preparation.4 Given that 3 million cataract surgeries are performed yearly in this country, an estimated 2,000 eyes could be saved by IC antibiotic prophylactic use. Big data from the Aravind Eye Hospital System5 and elsewhere are confirmatory for me and may be enough to convince others. Kaiser Permanente acted on its own big data6 prior to the evidence from India.
It is worth remembering that topical antibiotic prophylaxis is also an off-label practice and hardly without economic and biome-altering consequences. For surgeons who wish to use IC prophylaxis and do not choose a compounding route, Dr. Arshinoff has provided a detailed how-to guide; it is a template that I believe has benefited my patients (see Dr. Arshinoff’s Method of Preparation).
ASCRS has announced its intention to perform a multicenter randomized prospective trial that will require more than 75,000 patients to reach significance. This will hopefully provide a definitive answer and lead to uniform adoption of intracameral antibiotic prophylaxis. I hope most surgeons will not wait for the results far into the future.
CRST and I invited several well-known experts in our field to interpret the insights provided by the recent meta-analysis and to comment on their own practices.
—Lisa Brothers Arbisser, MD

FRANCIS S. MAH, MD
Nobody is going to perform a clinical trial comparing IC cefuroxime, moxifloxacin, and vancomycin to see if one of these agents is superior to the others. A meta-analysis of the literature is the next best thing; it helps to decrease the biases present in individual articles to uncover general findings.
The main take-away messages of the meta-analysis by Bowen and colleagues are as follows.3 In terms of the volume of studies and the volume of patients, IC cefuroxime has the greatest amount of support in the literature and vancomycin the lowest. Of the three agents, cefuroxime was the least effective, although it was more efficacious than topical drops. Moxifloxacin was more effective than cefuroxime and was associated with less toxicity. Most effective of the three agents was vancomycin, but it has been associated with hemorrhagic occlusive retinal vasculitis (HORV). Interestingly, topical medications did not appear to be of benefit in the meta-analysis.
My preferred IC agent is moxifloxacin. I am not using IC vancomycin for two reasons. First, HORV is a devastating complication, potentially worse than the endophthalmitis the antibiotic was meant to prevent. Second, the AAO and the Centers for Disease Control issued guidance against using this medication as a prophylactic agent.
ESTABLISHING TRUE ALLERGY
IC anaphylaxis has been associated with cephalosporins such as cefuroxime and cefazolin. At most, approximately 10% of patients who are allergic to penicillin are also allergic to cephalosporins. A history of penicillin allergy would therefore raise my concern over the use of IC cefuroxime. Compared with beta-lactam antibiotics such as the cephalosporins, an advantage of fluoroquinolones is that they are generally associated with fewer allergies.
The first step to establishing true allergy is the history. To my mind, patients who state that some pills upset their stomach in the past do not have a true allergy. If they say they developed hives or a rash, then I am more cautious. Because I work in a clinic, urgent care is nearby. In these cases, I will therefore instill a single drop of a fluoroquinolone in the patient’s eye and observe him or her for a reaction in the clinic. If nothing happens, I will plan to use IC moxifloxacin on the day of surgery. Obviously, a history of anaphylaxis is a clear contraindication to a medication.
If a patient has an allergy to fluoroquinolones but not to beta-lactam antibiotics, I will use cefazolin 2.5 mg in 0.1 mL instead of moxifloxacin.
ACCESSING IC ANTIBIOTICS
More US cataract surgeons would use IC antibiotics if an FDA-approved drug were available. Because that is not the case, one option is to obtain an IC formulation from a compounding pharmacy such as Leiters, a hospital pharmacy, or a company such as »Imprimis Pharmaceuticals or Ocular Science.
A second option is either to dilute commercially available moxifloxacin or to use it straight out of the bottle. The literature describes using anything from 50 to 500 mg in 0.1 mL at the end of surgery. As far as I know, there have been no published reports about using dilute generic moxifloxacin, and »Moxeza (moxifloxacin HCl ophthalmic solution 0.5%, Alcon) should definitely not be injected intracamerally.

DAVID F. CHANG, MD
The recent meta-analysis by Bowen and colleagues pools data from 17 studies published over the past 2 decades. Apart from the ESCRS randomized clinical trial,1 these were all observational studies. When pooled, however, more than 925,000 eyes can be analyzed, providing a large amount of data supporting the safety and efficacy of routine IC antibiotic prophylaxis for phacoemulsification.
One of the more interesting aspects is the comparison of different IC antibiotic agents. The meta-analysis includes a large observational study of IC moxifloxacin from the Aravind Eye Hospital System that my colleagues and I published in 2017, which significantly expands the pooled data for this drug.5 The comparative endophthalmitis rates were 0.033% for cefuroxime, 0.015% for moxifloxacin, and 0.011% for vancomycin. This suggests that both moxifloxacin and vancomycin provide better coverage than cefuroxime. The data also indicate that adding topical antibiotic prophylaxis may provide no benefit over IC antibiotic prophylaxis alone.
A joint task force formed by the ASCRS and the American Society of Retina Specialists, which I cochaired, concluded that HORV was a type III hypersensitivity to vancomycin.7 Although we suggested that the use of IC vancomycin should be left to the individual surgeon’s discretion, the AAO Cataract Preferred Practice Patterns and the FDA have since discouraged using IC vancomycin because of the risk of HORV. I personally used IC vancomycin routinely for 18 years without any known complications. Should a case of HORV ever occur, however, these strong pronouncements would make it difficult to defend the off-label use of IC vancomycin when alternative antibiotics were available.
Based on our Aravind studies, I have switched to IC moxifloxacin supplied by a 503B compounding pharmacy. Imprimis Pharmaceuticals and Leiters are the largest 503B outsourcing facilities that can supply moxifloxacin for IC injection.

RICHARD KENT STIVERSON, MD
The meta-analysis by Bowen and colleagues confirms the efficacy of IC antibiotics in preventing postcataract surgery endophthalmitis,3 as shown in the very large ESCRS,1 Kaiser,6 and Aravind studies.5 Although I am not qualified to comment on the statistical rigor of this meta-analysis, rigor is crucial and seems to be well documented in this study. The number of meta-analyses in medicine has increased markedly from 334 worldwide in 1991 to 5,000 in 2012 and 10,000 in 2017. It is important to note that meta-analyses are only as good as the trials included; in other words, including large numbers of low-quality studies does not make a meta-analysis more valid. Bowen and colleagues excluded several IC studies from their meta-analysis, which gives me more confidence in their findings.
Prospective randomized clinical trials remain the gold standard for evidence-based medicine, but their prohibitive cost is such that high-quality, reliable meta-analyses will assume increasing importance in answering doctors’ big questions. Meta-analysis gave rise to big data.
In my opinion, changing statistical significance to P < .005 would make a lot of published research more valid and worthier of consideration. Although a higher threshold for statistical significance might increase the chance of a false negative, multiple studies—even with smaller sample sizes—would reduce the chance of false positives. I highly recommend that readers watch the video, “Is Most Published Research Wrong?” by Derek Muller, BSc, PhD (bit.ly/2bbI6oF). Even randomized, prospective studies should be regarded with skepticism when the sample size is small and significance is based on a probability of less than 0.05.
I do not believe the work by Bowen and colleagues will change US cataract surgeons’ IC choices. Cefuroxime will not be widely adopted in the United States because of the risk of anaphylaxis and bacterial resistance profiles that are significantly different from those in Europe (in part because of antibiotic abuse in the United States). Kaiser started with cefuroxime because that was the antibiotic of the preexisting studies, but many Kaiser doctors have switched to moxifloxacin. The specter of HORV is such that most ophthalmologists have abandoned or will abandon IC vancomycin. Moxifloxacin will be the most commonly used IC antibiotic in the United States.
I stopped using topical antibiotics a year ago. Although I understand the belt-and-suspenders approach of using both topical and IC antibiotics that many surgeons advocate, there is simply no evidence to support it. Moreover, a large number of cataract surgery patients receive anti-VEGF injections or will in the future. The retina literature suggests that repeated prophylactic topical antibiotics might be contraindicated.8 A week of topical antibiotic prophylaxis after cataract surgery would certainly fall under this concern.
I use the same formulation as Dr. Arshinoff (see Dr. Arshinoff’s Method of Preparation below). Like him, I perform immediately sequential bilateral surgery. The importance of IC antibiotic prophylaxis cannot be overstated. His formulation allows more volume at a desired concentration so that most of the aqueous can be replaced with moxifloxacin solution. There is usually enough left over that I can also perform some stromal hydration of the incisions. That said, the success of staggering numbers of patients in the Aravind study,5 with such small volumes of moxifloxacin, suggests that my concerns are unfounded.
Dr. Arshinoff’s Method of Preparation
SUPPLIED
Moxifloxacin HCl ophthalmic solution 0.5% (Vigamox, Alcon) = 500 µg/0.1 mL
GOAL
150 µg/0.1 mL (dilution: 3 parts Vigamox + 7 parts BSS [Alcon])
In other words, to achieve 150 µg/0.1 mL, simply dilute the eye drops to 30% concentration of supplied Vigamox
METHOD
Inject 0.3 to 0.4 mL Vigamox 150 µg/0.1 mL at the end of the case = 450 to 600 µg → 1.0 to 1.2 mg/mL in the anterior chamber (AC).
Essentially, this is an exchange of most of the newly pseudophakic AC volume (0.5 mL) with the Vigamox solution. The volume indicated is what is likely left in the AC at the end of surgery.
DETAILED INSTRUCTIONS
1. From a new bottle of Vigamox, 3 mL is withdrawn into a 12-mL syringe with a sterile needle.
2. From a new 15-mL bottle of BSS, 7 mL is withdrawn into the same syringe. The two substances will be mixed by the turbulence of aspiration and rolling the syringe. The circulating nurse injects 0.8 mL of the Vigamox solution into a medicine cup on the surgical tray.
3. The scrub nurse draws up 0.6 mL of the Vigamox solution into a tuberculin syringe to hand to the surgeon.
4. The surgeon expels 0.1 mL to be sure there are no bubbles and then injects 0.3 to 0.4 mL via the sideport incision, as the last step of cataract surgery, under the distal edge of the capsulorrhexis. Then, as the eye is exited, a final spurt of injection is used at the incision to hydrate the incision and ensure the AC is left pressurized (Figures 1 and 2). This is a planned exchange of most of the AC contents and is therefore easy to do.
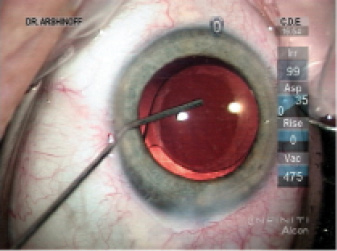
Figure 1. Via the sideport incision, Dr. Arshinoff administers an intracameral injection of moxifloxacin. He favors a hockey stick cannula because he can perform the injection slowly, thus deepening the capsular bag and allowing him to rotate the IOL slightly to the desired alignment. He continues the injection as the instrument exits the eye, ensuring an exchange of most of the aqueous with the moxifloxacin solution and pressurization of the AC. If concerned that the sideport incision will leak, Dr. Arshinoff will hydrate it prior to injecting the moxifloxacin, but usually he simply hydrates the incision as the cannula exits the eye.
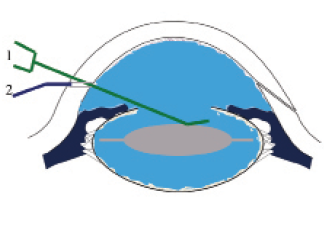
Figure 2. During the injection, the cannula (1, green) depresses the IOL slightly so that the moxifloxacin solution bathes the IOL within the capsular bag. As a sequestered space, the capsular bag is an excellent location for bacterial growth. The surgeon performs final hydration of the incision (2, purple) while removing the cannula from the eye.
PERSONAL EXPERIENCE
Dr. Arshinoff has used variations of this method in more than 8,700 cases and observed no toxicity to date.

STEVE A. ARSHINOFF, MD, FRCSC
One of the great strengths of the research by Bowen and colleagues is that it included observational studies as well as the sole randomized controlled study in this field,1 yielding a much broader depth of information than is available from the recent Cochrane review on the same subject.9 Bowen and colleagues concluded that, whether or not postoperative topical antibiotics were used, IC antibiotics all achieved statistically significant decreases in rates of infection, with no agent being statistically significantly better than the other two. (Statistical analysis also failed to demonstrate any additional benefit of postoperative topical antibiotics.)
The study by Bowen and colleagues is excellent, but the problem with meta-analyses is that they must, by their methodology, group data from different studies conducted at different times and analyze this aggregate data. Although this method produces useful results, the best understanding and conclusions come from studying the articles successively and rereading previous ones to discover what errors might have been made in each study and corrected by a later author or group. For example, two studies from Japan failed to demonstrate a statistically significant benefit of IC moxifloxacin, but when they are read carefully, it is because subtherapeutic doses of the drug were used.10,11 The sequential analysis approach thus gradually clarifies the answers to the questions all of us really want to ask and is more useful but much more time-consuming for the reader than meta-analyses. I will address some of these questions here.
AT A GLANCE
- A meta-analysis compared the safety and efficacy of intracameral cefuroxime, moxifloxacin, and vancomycin in preventing endophthalmitis after phacoemulsification cataract surgery.
- The meta-analysis pooled data from 17 studies published over the past 2 decades and included more than 925,000 eyes. This large amount of data supported the safety and efficacy of routine IC antibiotic prophylaxis.
DO IC ANTIBIOTICS WORK?
Adding up studies on the IC administration of cefuroxime, moxifloxacin, and vancomycin for cataract surgery endophthalmitis prophylaxis yields an aggregate of more than 6 million surgeries in the database. Every study (except two in which too low a drug dose was used10,11) demonstrated a reduction in postoperative endophthalmitis cases of about 80% when IC prophylaxis was used. The efficacy of IC antibiotics is no longer in question.
WHICH IC ANTIBIOTIC IS BEST?
In terms of efficacy, cefuroxime, moxifloxacin, and vancomycin all produce similar reductions in the incidence of postoperative endophthalmitis. As the database grows, I expect moxifloxacin to turn out to be the best—but only when the sample size becomes huge because the difference in efficacy is small. The use of cefuroxime is of concern because dilution errors have been shown to cause severe ocular damage.12 Moxifloxacin is much easier to dilute, and no complications from a dilution error have ever been reported. Another drawback to cefuroxime is that, as a relative of penicillin, it carries a much higher risk of allergy than the other two drugs. Vancomycin has recently been associated with a small risk of the devastating allergic complication of HORV. With vancomycin, it thus appears that we are trading one rare devastating risk for another. Moxifloxacin is therefore the safest of the three drugs.
Also worth noting is that data from the Swedish national database recently revealed that failures with IC cefuroxime are often Enterobacter cases, which are difficult to treat and usually result in devastating blindness.13 In contrast, failures with moxifloxacin are generally easy-to-treat cases of Staphylococcus, and these patients generally retain good vision. Furthermore, Libre and Mathews recently demonstrated that only moxifloxacin is effective against all 18 strains of endophthalmitis isolates from the Bascom Palmer Eye Institute.14
SHOULD IC ANTIBIOTICS BE USED FOR IMMEDIATELY SEQUENTIAL BILATERAL CATARACT SURGERY?
Absolutely. In 2009, the International Society of Bilateral Cataract Surgeons issued a recommendation that IC antibiotics be used for immediately sequential bilateral cataract surgery. The society’s study of endophthalmitis after bilateral cataract surgery found that, in more than 125,000 eyes, the infection rate when IC antibiotics were used was 1:16,890, the lowest infection rate reported in any study to date.15
I have used IC moxifloxacin for almost 9,000 consecutive eyes with no negative ocular side effects of the drug. I was the first in the world to use IC moxifloxacin and to propose its use generally. Early on, when I was using a low dose (100 µg in 0.1 mL), one patient developed a resistant strain of Staphylococcus epidermidis endophthalmitis, but with routine endophthalmitis treatment, visual acuity promptly improved to 20/25. I have since increased the dose I use and recommend to cover even the most resistant strains (see Dr. Arshinoff’s Method of Preparation). About 80% of my last 14,000 cataract surgeries have been immediately sequential bilateral cataract surgery.
1. Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33(6):978-988.
2. Arbisser LB. Safety of intracameral moxifloxacin for prophylaxis of endophthalmitis after cataract surgery. J Cataract Refract Surg. 2008;34(7):1114-1120.
3. Bowen RC, Zhou AX, Bondalapati S, et al. Comparative analysis of the safety and efficacy of intracameral cefuroxime, moxifloxacin and vancomycin at the end of cataract surgery: a meta-analysis [published online ahead of print January 11, 2018]. Br J Ophthalmol. doi:10.1136/bjophthalmol-2017-311051.
4. Javitt JC. Intracameral antibiotics reduce the risk of endophthalmitis after cataract surgery: does the preponderance of the evidence mandate a global change in practice? Ophthalmology. 2016;123(2):226-231.
5. Haripriya A, Chang DF, Ravindran RD. Endophthalmitis reduction with intracameral moxifloxacin prophylaxis: analysis of 600,000 surgeries. Ophthalmology. 2017;124(6):768-775.
6. Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg. 2013;39(1):8-14.
7. Witkin AJ, Chang DF, Jumper JM, et al. Vancomycin-associated hemorrhagic occlusive retinal vasculitis: clinical characteristics of 36 eyes. Ophthalmology. 2017;124(5):583-595.
8. Bande MF, Mansilla R, Pata MP, et al. Intravitreal injections of anti-VEGF agents and antibiotic prophylaxis for endophthalmitis: a systematic review and meta-analysis. Sci Rep. 2017;7(1):18088.
9. Gower EW, Lindsley K, Tulenko SE, et al. Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery. Cochrane Database Syst Rev. 2017;2:CD006364.
10. Matsuura K, Suto C, Akura J, Inoue Y. Bag and chamber flushing: a new method of using intracameral moxifloxacin to irrigate the anterior chamber and the area behind the intraocular lens. Graefes Arch Clin Exp Ophthalmol. 2013;251(1):81-87.
11. Matsuura K, Miyoshi T, Suto C, et al. Efficacy and safety of prophylactic intracameral moxifloxacin injection in Japan. J Cataract Refract Surg. 2013;39(11):1702-1706.
12. Olavi P. Ocular toxicity in cataract surgery because of inaccurate preparation and erroneous use of 50mg/ml intracameral cefuroxime. Acta Ophthalmol. 2012;90(2):e153-154.
13. Monta P. Antibiotics revisited. Paper presented at: XXXV Congress of the ESCRS; October 8, 2017; Lisbon, Portugal.
14. Libre PE, Mathews S. Endophthalmitis prophylaxis by intracameral antibiotics: in vitro model comparing vancomycin, cefuroxime, and moxifloxacin. J Cataract Refract Surg. 2017;43(6):833-838.
15. Arshinoff SA, Bastianelli PA. Incidence of postoperative endophthalmitis after immediate sequential bilateral cataract surgery. J Cataract Refract Surg. 2011;37(12):2105-2114.




