CASE PRESENTATION
A 53-year-old woman who desires improved visual acuity is referred to you. The patient received hyperopic LASIK 3 years ago. Her operative records are unobtainable, however, so her preoperative parameters are unknown. She subsequently developed severe epithelial ingrowth and flap necrosis in her right eye. The patient underwent multiple surgical interventions by several physicians, including the primary surgeon. Ultimately, the corneal flap was removed, followed by amniotic membrane placement. The eye has re-epithelialized.
The patient’s UCVA measures 20/200 OD, and a manifest refraction of -12.25 +0.75 X 090º yields a BCVA of 20/60. With a scleral contact lens in place, the patient’s BCVA improves to 20/40, with glare testing of 20/70. The slit-lamp examination is significant for mild corneal haze and 1+ to 2+ nuclear sclerosis. A central pachymetry reading is 402 µm (Figures 1 and 2).
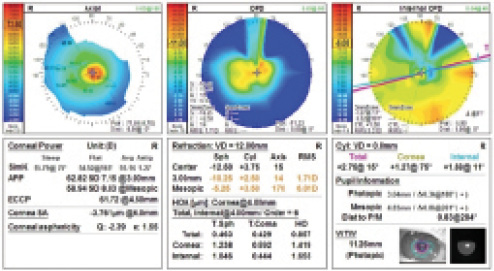
Figure 1. The OPD-Scan III (Nidek) shows severe central corneal steepening.
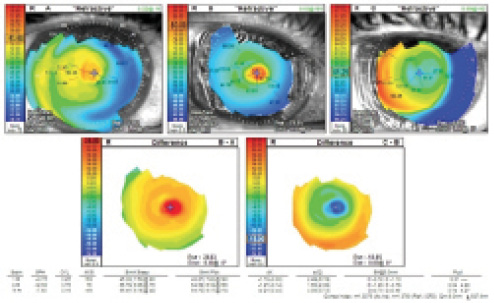
Figure 2. Corneal topography prior to flap removal (A), after flap removal (B), and 8 months after flap removal (C). The difference map comparing Figure 1A with Figure 1B shows severe steepening of the cornea (D), whereas the difference map comparing Figure 1B with Figure 1C does not (E).
Meibomian gland dysfunction (MGD) has reduced the patient’s contact lens wear to 1 to 2 hours per day. She has received maximum pharmaceutical and systemic intervention, and she has undergone two treatments with the »LipiFlow Thermal Pulsation System (Johnson & Johnson Vision) without improvement.
How would you proceed?
—Case prepared by Karl G. Stonecipher, MD

MARK KONTOS, MD
This is a very challenging case for two reasons: (1) the possibly unstable cornea and (2) the difficult IOL power calculation. It would be nice to know the patient’s preoperative degree of hyperopia. Is the eye stable from a refractive and topographic standpoint? It would also be helpful to know the status of the contralateral eye. Usually, flap amputation does not induce much refractive change, so the severe corneal steepening is either the result of the primary LASIK procedure or of abnormal healing.
First, I would address the abnormal shape and potential instability of the cornea. I would recommend CXL to stop any potential progressive steepening of the cornea, followed by careful topography-guided PRK to reduce the irregular contour and central steepening. More than one PRK treatment might be necessary.
Once the cornea had stabilized and the irregular astigmatism had decreased, I would observe the patient for at least 6 months while continuing aggressive treatment of the ocular surface. After corneal stabilization, the patient could undergo surgery for the 2+ nuclear sclerotic cataract. An irregular cornea and higher-order aberrations make her a poor candidate for a multifocal lens. Typically, a nonaspheric monofocal or accommodating IOL maximizes quality of vision after hyperopic LASIK. Given this patient’s complicated refractive status, intraoperative aberrometry could be helpful in determining the most appropriate IOL power, but obtaining accurate readings with the ORA System (Alcon) might prove difficult in this case.
Preoperatively, I would counsel the patient about her potential need for glasses or contact lenses after surgery. I would also inform her that she might require an IOL exchange and/or further corneal refractive surgery.

MITCHELL A. JACKSON, MD
I have amputated two flaps after LASIK to manage severe epithelial ingrowth that was unresponsive to standard management. Removing the flap did not induce a severe refractive change in either case, so I presume the alteration here is related instead to the hyperopic treatment. Details on the patient’s treatment prior to the original LASIK surgery would be helpful.
Key to management in this case is achieving corneal stability. CXL is an option, but I am not sure how much stability it would provide to the eye of a 53-year-old woman.1 The pachymetry reading of 402 µm might make a postoperative LASIK enhancement problematic. My first step would therefore be to remove the nuclear sclerotic cataract, which would provide a refractive solution to the patient’s ametropia and should improve her visual acuity. The challenge lies in selecting the IOL.
In patients who have undergone hyperopic LASIK, I typically favor the zero-aberration »MX-60 IOL (Bausch + Lomb). I would target -0.75 to -1.00 D of myopia. In addition to corneal topography, I would use all available online calculators and intraoperative aberrometry to assist my choice of IOL power. Although I would plan to perform cataract extraction in my standard manner, I would carefully mark the cornea preoperatively in case an intraoperative refractive surprise required me to change my choice of IOL. Corneal astigmatism currently measures 1.27 D, but that could change after the lens is removed. I would be prepared in case intraoperative aberrometry proved challenging.
Prior to surgery, I would thoroughly discuss possible postoperative outcomes with the patient. If I encounter a refractive surprise, for instance, I would be inclined to consider a laser enhancement with CXL. I would also inform the patient that reducing her refractive error closer to emmetropia will give her more options as far as contact lenses.

WHAT I DID: KARL G. STONECIPHER, MD
Many patients are referred to me for refractive surgery complications, but this case represents one of the greatest challenges I have faced after LASIK. Many excellent surgeons had already seen and treated the patient, and numerous interventions had failed to resolve her problems. To my mind, this case exemplifies the negative effects of undertreated MGD on LASIK outcomes. The corneal steepening induced by the hyperopic LASIK procedure exacerbated her preexisting evaporative dry eye disease. The corneal melting and epithelial ingrowth were directly related to peripheral marginal keratitis postoperatively.
Flap removal after LASIK is a straightforward procedure. I observed the patient for more than 9 months until the cornea stabilized. She was able to wear a scleral contact lens with success, but the cataract progressed. Her visual acuity declined, and her contact lens wear time decreased despite aggressive intervention for MGD, including intense pulsed light therapy, low-level light therapy, and LipiFlow treatment.
The patient and I decided to proceed with cataract surgery. Preoperative testing was extensive to assist with IOL selection. I assessed corneal curvature using the Holladay software on the »Pentacam (Oculus Optikgeräte) and studied corneal maps obtained with the »OPD-Scan III. I used the »Lenstar LS900 (Haag-Streit), »IOLMaster (Carl Zeiss Meditec), and ultrasound measurements to obtain weighted averages of the IOL power calculations. I supplemented those readings with results obtained from the ASCRS online software for hyperopia, the Barrett formula, and the Haigis formula for postoperative hyperopic LASIK.
After presoftening the lens and performing the capsulotomy with a femtosecond laser, I used a blade to manually create an on-axis incision. Intraoperatively, I used the ORA System statically and dynamically. Cataract surgery was uneventful.
Postoperatively, the patient has a UCVA of 20/25, and her manifest refraction is essentially plano. Nevertheless, she wears a contact lens when she wants to see her best because she finds that it improves the quality of her vision. The patient has since undergone cataract surgery on her left eye. She manages her evaporative dry eye disease with cyclosporine ophthalmic emulsion 0.05% (»Restasis; Allergan) and lid hygiene (Avenova; NovaBay Pharmaceuticals) twice per day.
1. Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009;35(8):1358-1362.




