

Laser vision correction (LVC) procedures for hyperopic and myopic eyes differ in several fundamental ways. First, the latent component of hyperopia can make both preoperative planning and the postoperative analysis of results more challenging. Second, the anterior chamber in hyperopic eyes is often too shallow to permit the implantation of a phakic IOL. Third, since its inception, hyperopic LVC has been a subject of controversy because the ablation profile is more complex than that for treating myopia (see Analysis of a Hyperopic Ablation Profile.
Analysis of a Hyperopic Ablation Profile
Hyperopic ablations steepen the central cornea by removing tissue in the midperiphery. This process produces a more pronounced change in corneal curvature than myopic ablations do. Compared to myopic LASIK, hyperopic LASIK yields a smaller effective optical zone and increases higher-order aberrations,1 and treatment centration is less forgiving, which can cause more problems with night vision and produce less predictable outcomes.2 This was especially true with early excimer laser platforms that used broad-beam or scanning-slit technology, had no eye tracker, or had no transition zone.
1. Plaza-Puche AB, Aswad A El, Arba-Mosquera S, Wróbel-Dudzinska D, Abdou AA, Alió JL. Optical profile following high hyperopia correction with a 500-Hz excimer laser system. J Refract Surg. 2016;32(1):6-13.
2. McGhee CN, Ormonde S, Kohnen T, Lawless M, Brahma A, Comaish I. The surgical correction of moderate hypermetropia: the management controversy. Br J Ophthalmol. 2002;86(7):815.
These three specific challenges led to restrictions on the use of hyperopic LASIK. For instance, in Germany, the Commission for Refractive Surgery does not recommend hyperopic laser ablation for the correction of more than 4.00 D of refractive error or more than 6.00 D of astigmatism.1 These ranges have not changed for more than a decade despite significant technological advances, which our colleagues and we believe warrant a reevaluation of LVC for hyperopia and hyperopic astigmatism.
STUDY DESIGN
We conducted a retrospective analysis of our clinical results with LASIK for hyperopia and hyperopic astigmatism performed with one of two excimer laser systems. A Technolas 217z100 (Bausch + Lomb) was used for surgeries performed from April 2015 to January 2018; a Teneo 317 model 2 (Bausch + Lomb) was used for surgeries performed from February 2018 to December 2020. All LASIK flaps were created with a VisuMax 500-Hz femtosecond laser (Carl Zeiss Meditec).
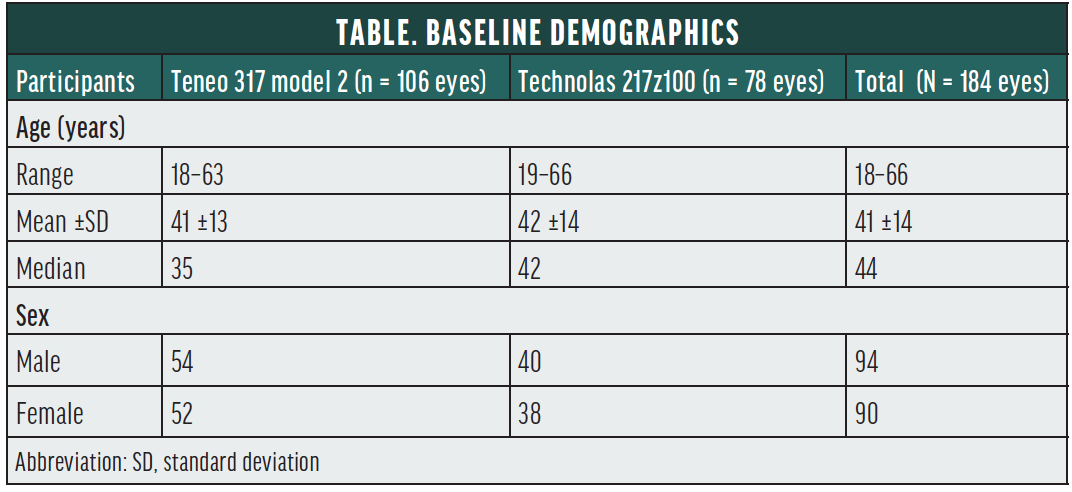
None of the eyes included in our study (N = 184) had preoperative ocular pathology or a history of surgical intervention. Eyes with a corrected distance visual acuity (CDVA) worse than 20/25 were excluded. The Table shows baseline demographics. Approximately half of the patients were in the presbyopic age group.
RESULTS
LASIK performed with the Teneo corrected 0.75 to 6.00 D of spherical error and 0 to -5.75 D of cylinder (Figure 1*). In this group, the preoperative mean refractive spherical equivalent at the spectacle plane was 2.34 D (range, 0.50–5.38 D), and the attempted mean spherical correction was 2.22 D (range, 0.50–4.88 D). LASIK procedures performed with the Technolas corrected 0.75 to 5.25 D of spherical error and 0 to -4.00 D of cylinder (Figure 2*). In this group, the preoperative mean refractive spherical equivalent was 1.92 D (range, 0.38–5.13 D), and the attempted mean spherical correction was 1.86 D (range, 0.38–5.00 D).
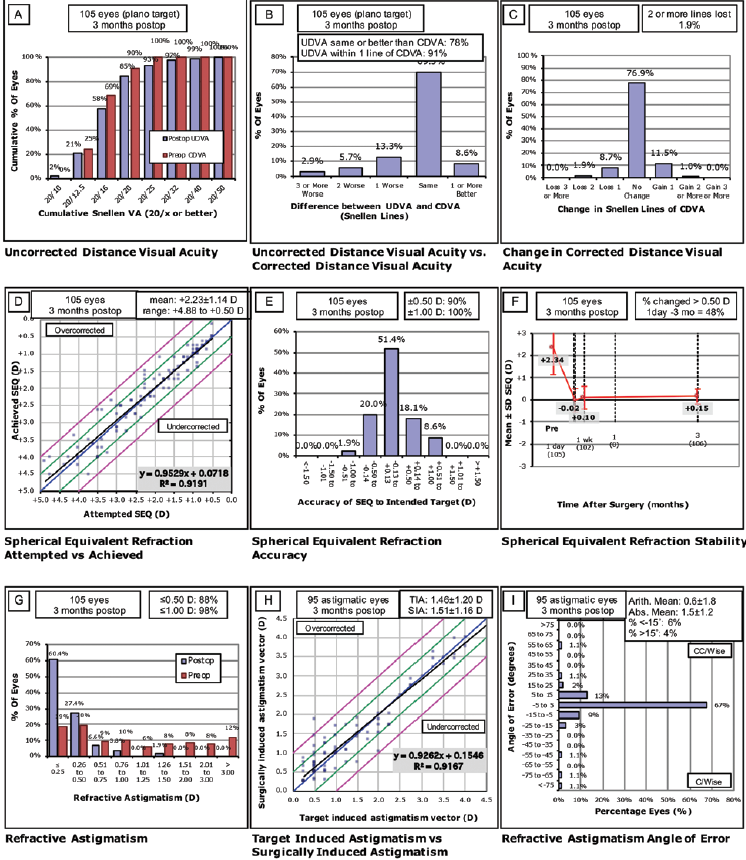
Figure 1. Postoperative results in eyes treated with the Teneo 317 model 2.*
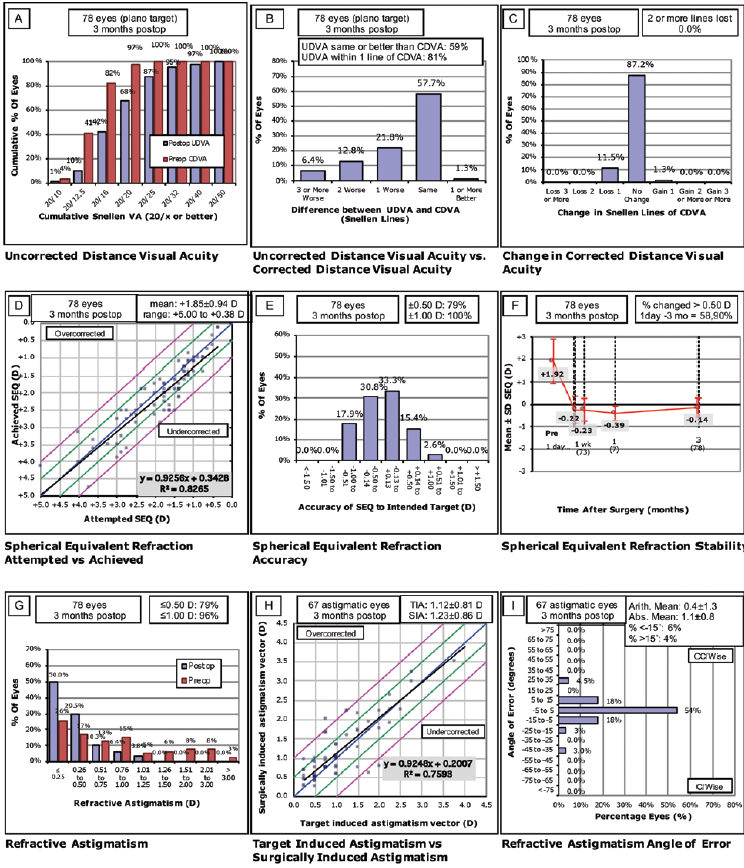
Figure 2. Postoperative results in eyes treated with the Technolas 217Z100.*
At 3 months, mean uncorrected distance visual acuity (UDVA) was 1.14 (decimal scale) in the Teneo group and 1.00 (decimal scale) in the Technolas 217z100 group. The mean efficacy index (mean postoperative UDVA/mean preoperative CDVA) was 0.97 in the Teneo group and 0.75 in the Technolas group. Mean safety index (mean postoperative CDVA/mean preoperative CDVA) was 0.99 in the Teneo group and 0.95 in the Technolas group.
Postoperative UDVA was the same as or better than preoperative CDVA in 75% of the eyes treated with the Teneo and 59% of the eyes treated with the Technolas. All eyes were within ±1.00 D of the intended target in both datasets, and 90% of the eyes in the Teneo group and 79% of the eyes in the Technolas group were within ±0.50 D of the intended target.
At 3 months, residual astigmatism outcomes were better in the Teneo group, although there was a trend toward slight undercorrection in eyes that required correction for high astigmatism. Further, there was a better correlation coefficient compared to the Technolas.
Only one eye received a second refractive treatment. The initial target for that eye was reached after the first treatment, which was with the Teneo. The patient’s preferences changed, however, and blended vision correction with relifting of the flap was performed 6 months later.
DISCUSSION
The incidence of myopia is increasing worldwide.2 Meanwhile, the prevalence of hyperopia, which increases with age, was recently reported to be 32% in Europe among patients who were 35 to 74 years of age.3 Treatment options for hyperopic patients are limited, especially for prepresbyopic patients. Safe, predictable surgical means of correcting hyperopia with or without astigmatism in patients who have clear lenses and retain accommodative ability are desirable.
In our study, we found that visual and refractive outcomes were excellent 3 months after LASIK for the correction of hyperopia and hyperopic astigmatism using two modern excimer laser systems. Furthermore, the duration of follow-up lasted for up to several years, and the low rate of enhancements suggests that the regression of corrective effect was not a significant problem.
Possible reasons why our results were better than those reported in earlier studies may be improvements in eye trackers, higher laser repetition rates that have decreased treatment times and dehydration, and better ablation profiles that incorporate wider transition zones that have resulted in less masking of the ablation by the epithelium.
We included treatments that were beyond the range recommended in Germany by the Commission for Refractive Surgery. We performed such treatments, however, only after carefully determining that the postoperative maximum keratometry would not be steeper than 51.00 D and that the preoperative epithelial thickness was at least 50 µm to avoid an unstable tear film and an epithelial breakdown at the corneal apex postoperatively. Because of the smaller effective optical zone and the potential for regression after a hyperopic laser treatment, our treatment range in this patient population was narrower than the range we use for myopic or myopic astigmatic ablations.
Our analysis found no need for a nomogram adjustment when transitioning from the Technolas 217Z100 to the Teneo 317 for the correction of hyperopia with or without astigmatism. Furthermore, our results and the findings of several recent publications on this topic with other modern laser systems4-6 suggest that technological advances have overcome previously reported disadvantages such as treatment decentration and regression.7,8 Our slightly better results with the Teneo 317 compared to its predecessor, the Technolas 217z100, may be due to incremental technological improvements.
Longer-term analysis is warranted to determine if hyperopic regression occurs. That said, the earliest treatments reported here occurred 6 years ago, and no patient has returned because of regression. However, some eyes have received subsequent cataract surgery.
CONCLUSION
Based on our results, LASIK will continue to be our treatment of choice for the correction of low to moderate hyperopia with or without astigmatism in patients with clear lenses.
1. Kohnen T, Steinwender G. Laser-in-situ-keratomileusis mit mikrokeratom oder femtosekundenlaser. Ophthalmologe. 2017;114(7):661-665.
2. Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-1042.
3. Wolfram C, Höhn R, Kottler U, et al. Prevalence of refractive errors in the European adult population: the Gutenberg Health Study (GHS). Br J Ophthalmol. 2014;98(7):857-861.
4. Reinstein DZ, Carp GI, Archer TJ, Day AC, Vida RS. Outcomes for hyperopic LASIK with the MEL 90 excimer laser. J Refract Surg. 2018;34(12):799-808.
5. Demir G, Sucu ME, Yıldırım Y, et al. Long-term assessment of visual and refractive outcomes of laser in situ keratomileusis for hyperopia using the Amaris 750S excimer laser. J Fr Ophtalmol. 2019;42(7):703-710.
6. Arba-Mosquera S, de Ortueta D. LASIK for hyperopia using an aberration-neutral profile with an asymmetric offset centration. J Refract Surg. 2016;32(2):78-83.
7. Alio JL, Pachkoria K, El Aswad A, Plaza-Puche AB. Laser-assisted in situ keratomileusis in high mixed astigmatism with optimized, fast-repetition and cyclotorsion control excimer laser. Am J Ophthalmol. 2013;155(5):829-836.
8. El-Naggar MT, Hovaghimian DG. Assessment of refractive outcome of femtosecond-assisted LASIK for hyperopia correction. Electron Physician. 2017;9(3):3958-3965.




