Individuals with cataracts also often have glaucoma because increasing age is a risk factor for the development of both.1 There is growing evidence that cataract surgery can lower IOP in eyes with glaucoma.2 The role of cataract surgery as an option to control IOP remains controversial in eyes with open-angle glaucoma (OAG). The procedure’s efficacy, however, is well established for eyes with angle-closure glaucoma (ACG),2 largely owing to greater changes in the anterior segment configuration of eyes with a closed versus an open anterior chamber angle. Evidence from the randomized controlled Effectiveness of Early Lens Extraction for the Treatment of Primary Angle-Closure Glaucoma (EAGLE) trial supports the IOP-lowering efficacy of clear lens extraction in eyes with primary ACG (PACG).3
AT A GLANCE
- The refractive outcome of cataract surgery tends to be less predictable in eyes with angle-closure glaucoma than in eyes without glaucoma and eyes with other forms of the disease.
- A recent study suggests that the presence or absence of peripheral anterior synechiae may influence postoperative refractive outcomes in patients with primary angle-closure glaucoma.
Another consideration with cataract surgery on eyes with glaucoma is that refractive outcomes can be less accurate than in eyes without the disease,4,5 and the difference seems to be more pronounced in eyes with ACG.5,6 We recently studied the influence of peripheral anterior synechiae (PAS) on the refractive outcome of cataract surgery in eyes with PACG. This article summarizes our results.7
REFRACTIVE PREDICTABILITY
Manoharan and colleagues found that the incidence of a refractive surprise after cataract surgery was higher in eyes with glaucoma than in control eyes (40.3% vs 29.9%).6 The odds ratios of a refractive surprise in eyes with OAG, ACG, and pseudoexfoliation glaucoma were 1.90, 14.54, and 7.27 (all P < .05), respectively.5 Refractive outcomes after clear lens extraction in eyes with PAC and an IOP greater than 30 mm Hg and in eyes with PACG were also suboptimal in the EAGLE trial.
Kang et al retrospectively compared the refractive predictability of phaco cataract surgery in PACG and normal eyes.8 IOL power calculations were inaccurate in 50% of the PACG group compared to 27% of the control group. Among PACG eyes, 21% experienced a hyperopic shift, and 29% experienced a myopic shift.
A retrospective Korean study found no significant difference in the refractive predictability of cataract surgery in eyes with OAG and ACG.9 A large lens vault, however, was shown to be predictive of an unsatisfactory postoperative refractive outcome. In contrast, Kim et al found that the postoperative change in refractive error was greater in ACG eyes compared to eyes without glaucoma or OAG, and the direction of the refractive shift varied.10 Preoperative lens thickness also correlated with greater postoperative absolute changes in refractive error. Additionally, Seo et al found that IOL power calculations for PAC and PACG eyes could be inaccurate; the investigators reported a tendency toward hyperopia and found that the preoperative relative lens vault predicted the postoperative refractive outcome.11
Refractive outcomes in eyes with ACG tend to be less predictable because of their anatomical features, including a shallow anterior chamber, a thickened and anteriorly positioned lens, weak zonules, and a short axial length. The direction of the refractive shift (hyperopic or myopic) after phacoemulsification appears to vary and to depend on the magnitude of the influencing factors.
PERIPHERAL ANTERIOR SYNECHIAE
PAS are permanent adhesions between the iris and the corneoscleral region. They are an important risk factor for IOP elevation in PACG and one of the pathognomonic signs used to differentiate PAC/PACG from suspected PAC.12 PAS are also associated with anterior placement of the ciliary processes.13 In this regard, one may expect an IOL to be more anteriorly located in eyes with PAS.
To evaluate the effects of PAS on refractive outcomes, we recently conducted a retrospective, cross-sectional study of 70 patients with PACG who underwent cataract surgery.7
Effect of PAS on refractive outcomes. Patients were divided into two groups based on the presence or absence on preoperative gonioscopy of PAS—the PAS (+) and PAS (-) groups, respectively. The IOL power was calculated using the SRK/T, Hoffer Q, Haigis, and Holladay formulas. The mean absolute error (MAE) and predicted refractive errors were compared between PAS (+) and PAS (-) groups. In a subanalysis, we evaluated refractive error with regard to the extent of PAS.
The MAE was greater in the PAS (+) group (0.61–0.70 vs 0.33–0.45 D, all P < .05) with all four formulas. Refractive outcomes tended toward myopia in the eyes with PAS (-0.30 to -0.51 vs -0.05 to +0.24 D, all P < .05). The MAEs or predicted refractive errors, however, were not different irrespective of the extent of PAS (all P > .05).
There are several possible explanations for a less predictable refractive outcome and greater myopic shift in eyes with PAS. First, the PAS may be evidence of the structural difference between the two groups. Assuming no significant intergroup difference in other biometric variables, eyes with PAS may have abnormalities such as weak zonules or a large capsular bag. IOL instability due to these abnormalities could have induced a greater degree of refractive error in eyes with PAS. Increased choroidal thickness has also been reported to be associated with a myopic shift after cataract surgery in eyes with PACG.14 These various anatomic risk factors might have resulted in the PAS formation.
Second, deepening of the anterior chamber after surgery might have differed between the two groups. The presence of PAS might have limited posterior shifting of the IOL plane, which could explain the greater myopic shift experienced by eyes with PAS compared to eyes without PAS (Figure). Supporting this hypothesis is a report by Yoo et al that the trabecular–ciliary processes distance was shorter in PACG eyes with PAS than in PACG eyes without PAS. This suggests that anteriorly located ciliary processes might have influenced the development of PAS.13
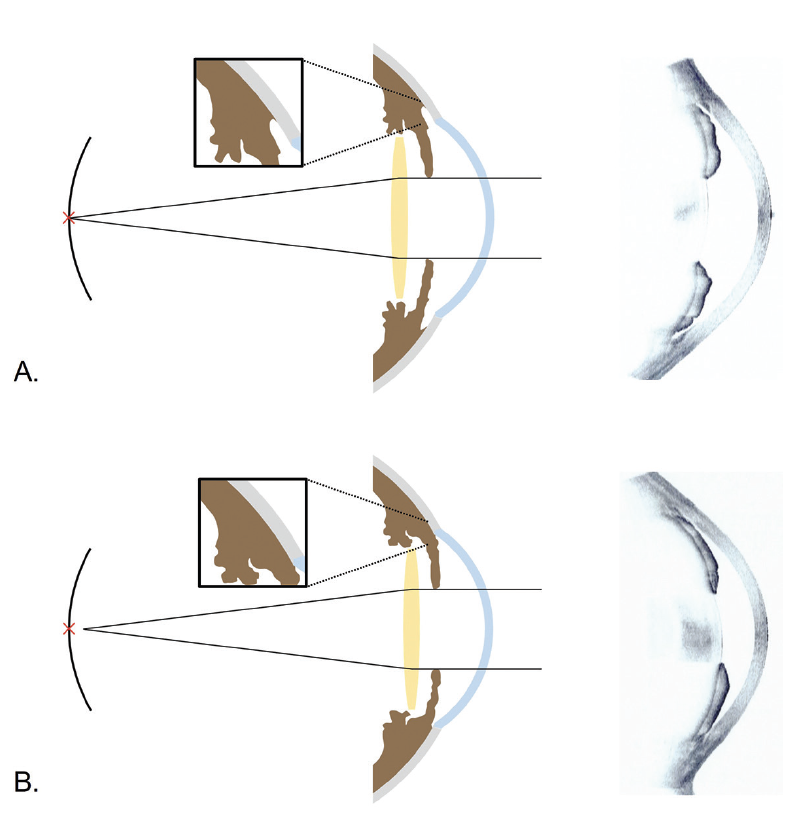
Figure. Possible mechanisms of refractive error after cataract surgery in PAC eyes without (A) and with (B) PAS and representative images of anterior segment OCT. Because anterior chamber deepening was limited by PAS, a myopic shift could occur. Reprinted from Lee TE et al.13
Limitations and implications. Our study is limited by its retrospective design and lack of postoperative PAS assessment. Additional studies on the extent of postoperative PAS and their effects on refractive outcomes are warranted to validate our findings.
CONCLUSION
Our study indicates that IOL power calculations can be less accurate in ACG eyes with PAS compared to ACG eyes without PAS. The presence or absence of PAS may influence postoperative refractive outcomes in patients with PACG.
1. Tseng VL, Yu F, Lum F, Coleman AL. Risk of fractures following cataract surgery in Medicare beneficiaries. JAMA. 2012;308(5):493-501.
2. Chen PP, Lin SC, Junk AK, Radhakrishnan S, Singh K, Chen TC. The effect of phacoemulsification on intraocular pressure in glaucoma patients: a report by the American Academy of Ophthalmology. Ophthalmology. 2015;122(7):1294-1307.
3. Azuara-Blanco A, Burr J, Ramsay C, et al; EAGLE study group. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. Lancet. 2016;388(10052):1389-1397.
4. Kugelberg M, Lundström M. Factors related to the degree of success in achieving target refraction in cataract surgery: Swedish National Cataract Register study. J Cataract Refract Surg. 2008;34(11):1935-1939.
5. Manoharan N, Patnaik JL, Bonnell LN, et al. Refractive outcomes of phacoemulsification cataract surgery in glaucoma patients. J Cataract Refract Surg. 2018;44(3):348-354.
6. Day AC, Cooper D, Burr J, et al. Clear lens extraction for the management of primary angle closure glaucoma: surgical technique and refractive outcomes in the EAGLE cohort. Br J Ophthalmol. 2018;102(12):1658-1662.
7. Lee TE, Yoo C, Kim YY. The effects of peripheral anterior synechiae on refractive outcomes after cataract surgery in eyes with primary angle-closure disease. Medicine (Baltimore). 2021;100(14):e24673.
8. Kang SY, Hong S, Won J, Seong GJ, Kim CY. Inaccuracy of intraocular lens power prediction for cataract surgery in angle-closure glaucoma. Yonsei Med J. 2009;50(2):206-210.
9. Kim YC, Sung MS, Heo H, Park SW. Anterior segment configuration as a predictive factor for refractive outcome after cataract surgery in patients with glaucoma. BMC Ophthalmol. 2016;16(1):179.
10. Kim KN, Lim HB, Lee JJ, Kim CS. Influence of biometric variables on refractive outcomes after cataract surgery in angle-closure glaucoma patients. Korean J Ophthalmol. 2016;30(4):280-288.
11. Seo S, Lee CE, Kim YK, Lee SY, Jeoung JW, Park KH. Factors affecting refractive outcome after cataract surgery in primary angle-closure glaucoma. Clin Exp Ophthalmol. 2016;44(8):693-700.
12. Choi JS, Kim YY. Relationship between the extent of peripheral anterior synechiae and the severity of visual field defects in primary angle-closure glaucoma. Korean J Ophthalmol. 2004;18(2):100-105.
13. Yoo C, Oh JH, Kim YY, Jung HR. Peripheral anterior synechiae and ultrasound biomicroscopic parameters in angle-closure glaucoma suspects. Korean J Ophthalmol. 2007;21(2):106-110.
14. Song WK, Sung KR, Shin JW, Kwon J. Effects of choroidal thickness on refractive outcome following cataract surgery in primary angle closure. Korean J Ophthalmol. 2018;32(5):382-390.