CASE PRESENTATION
A 27-year-old man seeking vision correction presented for an evaluation. The patient’s manifest refraction was -8.75 -1.00 x 179º = 20/16 OD and -8.25 -0.50 x 51º = 20/16 OS. Imaging with the Orbscan (Bausch + Lomb; Figure 1) was normal. On examination, the anterior segment anatomy of each eye appeared to be suitable for the implantation of a phakic posterior chamber IOL. The white-to-white (WTW) distance was 11.7 mm in each eye.
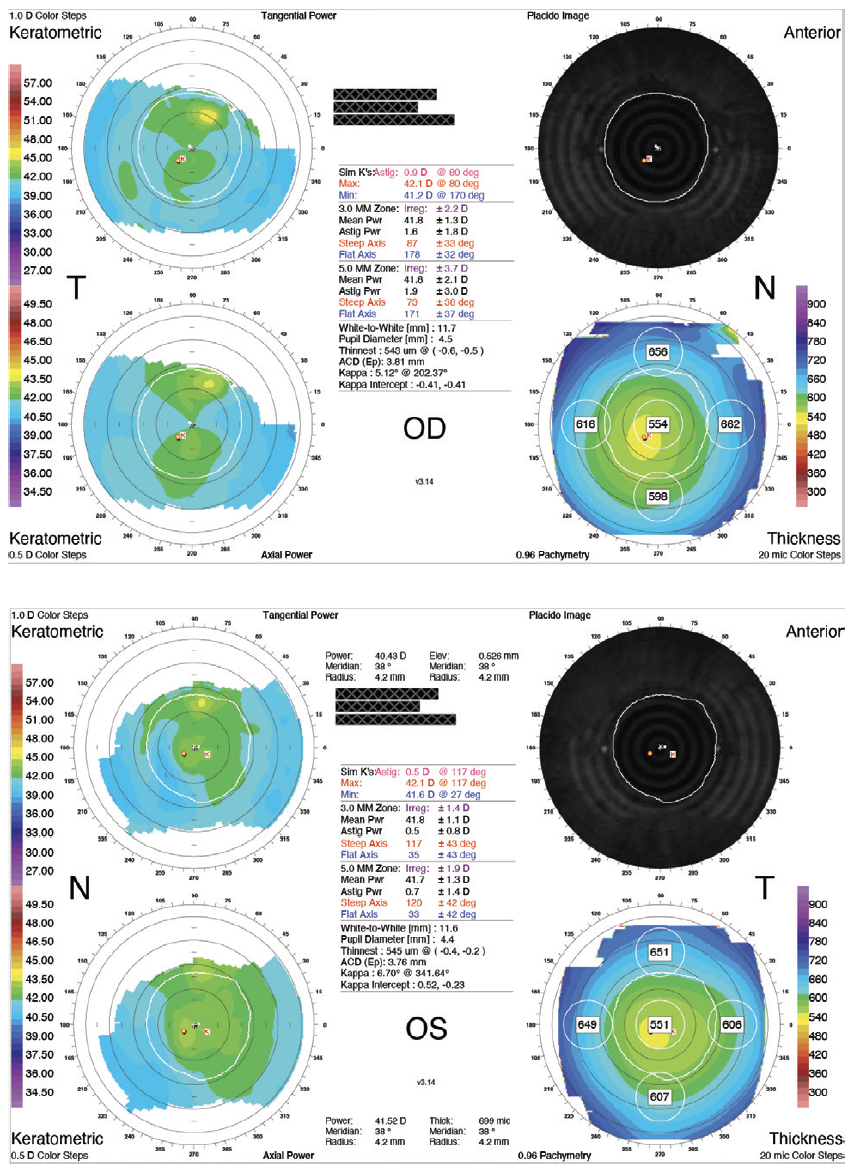
Figure 1. Preoperative Orbscan measurements.
An Evo Visian ICL (model V4c, STAAR Surgical) with a central hole (KS-AquaPort) and a diameter of 13.2 mm, as recommended by the manufacturer, was implanted in each eye. The procedures were performed 5 days apart. A -10.50 D lens was implanted in the right eye and a -9.50 D in the left eye.
The patient’s UCVA in each eye was excellent at 1 day, 1 week, and 1, 3, and 12 months. During the early postoperative period, ICL vaulting was average in the right eye but low in the left eye. Vaulting of both ICLs decreased from visit to visit.
At 12 months, the patient remained satisfied with his uncorrected distance visual acuity (20/12.5 OD and 20/16 OS). Vaulting of the ICLs was 371 µm OD and 77 µm OS, as measured with OCT (Spectralis, Heidelberg Engineering; Figure 2).
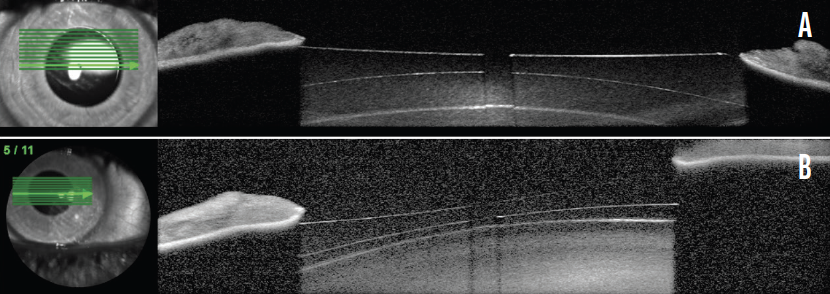
Figure 2. OCT scans show the Evo Visian ICL vault in the right (A) and left (B) eyes.
Figures 1 and 2 courtesy of Suphi Taneri, MD, FEBOS-CR
How would you proceed? Do you recommend exchanging one or both ICLs for a larger ICL? Do you have certain cutoffs for low and high vaulting? How do you choose an ICL size? Alternatively, would you recommend another treatment modality to this patient who is still happy with his vision?
—Case prepared by Suphi Taneri, MD, FEBOS-CR

ARTHUR B. CUMMINGS, MB CHB, FCS(SA), MMED(OPHTH), FRCS(EDIN)
Given the patient’s age, I want to avoid cataract formation at all costs. If he were closer to 60 years of age, I might recommend observation alone. He is 27 years old, however, so something must be done.
There is documented evidence that vaulting decreased at each visit, so a further reduction can be anticipated at the next visit. My cutoff vaults are a high of more than 1,000 µm and a low of less than 100 µm. To clarify, if, for example, vaulting is 1,100 µm or 80 µm for years without consequences for the endothelium or the crystalline lens, then I would likely recommend ongoing observation. Because the patient is young and a progressive reduction in vaulting has been documented, I would exchange the IOL in the left eye for a 13.7-mm ICL. It is interesting to note that there is no consensus on cutoffs among experts and that there are many patients with vaults of 50 µm who have clear crystalline lenses.
I choose the ICL size by using several devices that measure the WTW distance, but I feel most confident about the anterior segment OCT (AS-OCT) device MS-39 (CSO Italia) using the NK formula: optimal ICL size (mm) = 4.20 +0.719 (anterior chamber width in mm) +0.655 (crystalline lens rise in mm).
Anterior chamber width is defined as the distance between the scleral spurs on the nasal and temporal sides. Crystalline lens rise is defined as the anteroposterior distance between the anterior crystalline lens surface and the angle recess–to–angle recess line.
This strategy has worked well for me to date. Nevertheless, I am strongly considering acquiring a very high-frequency digital ultrasound (VHFDU) unit (ArcScan Insight 100, ArcScan) and using it for ICL sizing because the published literature suggests that this device is the most accurate method available.1

ERIK L. MERTENS, MD, FEBOPHTH
The patient had a successful refractive result during the first postoperative year. The ICL model selected eliminates the iridotomy/iridectomy step, which makes the procedure as efficient as LASIK. I implanted the first Evo Visian ICL in Europe in June 2011,2 which gives this phakic IOL a track record of almost 11 years.
Alfonso et al reported that vaulting of the ICL decreases over time,3 so the finding in the current case is no surprise. The aforementioned study found a continuous reduction in the central vault over time. The reduction was greatest during the first 6 months after surgery and then tended to slow over time.
Unlike with previous ICL models, only a small number of cases of anterior subcapsular lens opacities have been reported with the Evo and Evo+ (model V5) Visian ICLs when the central vault is less than 100 µm.4 I treated 43 eyes in which the postoperative vault was less than 100 µm and the follow-up period was at least 7 years. Only one (2.3%) of these eyes developed an anterior subcapsular lens opacity, supporting the findings of the aforementioned study.3 I therefore would not exchange the ICL in the left eye but would recommend routine follow-up every 6 months to evaluate the situation.
I have no cutoff for a low vault because the rate of complications with the Evo Visian ICL has been low. This may be attributed to the KS-AquaPort, which forces aqueous to flow between the crystalline lens and the implant.5 I tend to be more careful about vaults greater than 1,000 µm. In this situation, I discuss with the patient the signs and symptoms of complications and measure the anterior chamber angle to determine the risk of IOP elevation. If the implant is not a toric model, simply rotating it toward the vertical axis can relieve the problem and reduce the vault significantly,6 eliminating the need for an IOL exchange.
Historically, the choice of Evo Visian ICL size has been dictated by the eye’s WTW distance. Modern alternatives are to use angle-to-angle and/or scleral spur–to–scleral spur distances measured with AS-OCT and the crystalline lens rise.7-9 I use the MS-39 and the IOLMaster 700 (Carl Zeiss Meditec), and I incorporate other measured variables such as corneal volume, the anterior hemi-meridian of the flat and steep keratometry readings from the MS-39, and the lens thickness measurement from the IOLMaster 700. All of these parameters are entered into a spreadsheet to calculate the lens size that should achieve an ideal postoperative vault. The formula is under evaluation and will probably be published later this year.

DAN Z. REINSTEIN, MD, MA(CANTAB), FRCSC, DABO, FRCOPHTH, FEBO, PGDIP CRS, CERT LRS
I would monitor the patient closely because an ICL exchange is not free of risk. Neither, however, is cataract surgery on a young patient with high myopia10,11 in the context of the lower incidence of cataract formation reported with the Evo Visian ICL, even if the vault is low, compared to earlier ICL models.
I would ask the patient to return for observation every 6 months initially and then annually. The pupil would be dilated and the lens examined to determine if a cataract is forming.
Based on the case presentation, the central vault was measured, but this is not the minimum lens separation. The thickest portion of a highly myopic, concave ICL resides behind the iris and thus cannot be imaged by OCT. The only way of determining the physiologic minimum lens separation is with VHFDU because the thickest midperipheral lens zone is located behind the iris (Figure 3). OCT does not work in this situation because pupillary dilation to access the zone causes the ICL to move forward.12-14 Information on the minimum lens separation in an undilated physiologic state is required. VHFDU also allows the lens separation to be considered over the entirety of the midperipheral zone. The lens vault map shown in Figure 4 is derived from multimeridional scanning using the ArcScan Insight 100.
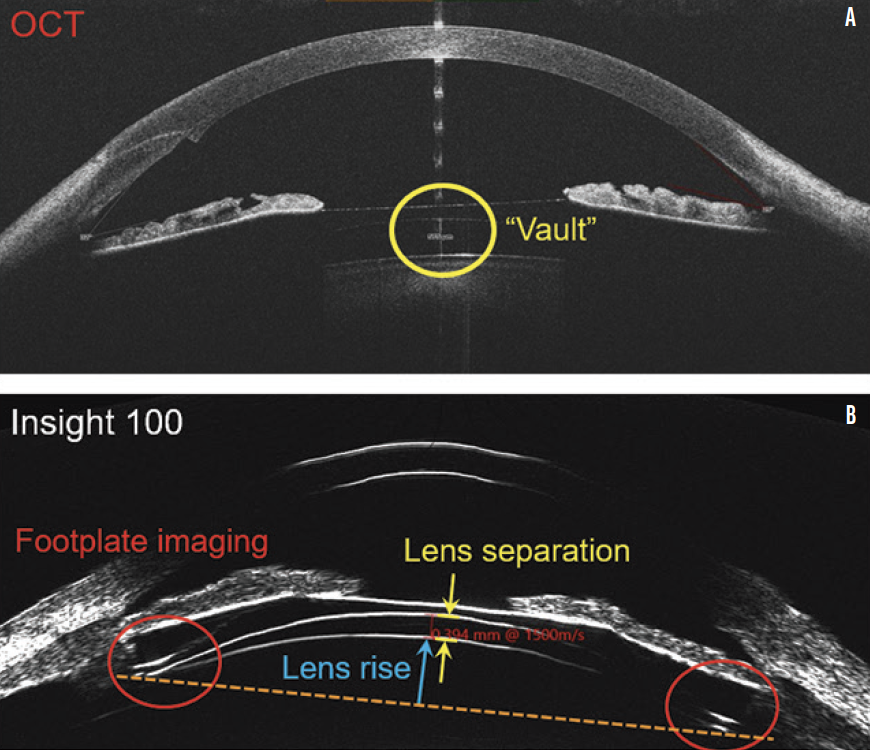
Figure 3. OCT (MS-39, A) and VHFDU (ArcScan Insight 100, B) scans of an eye that received an Evo Visian ICL.
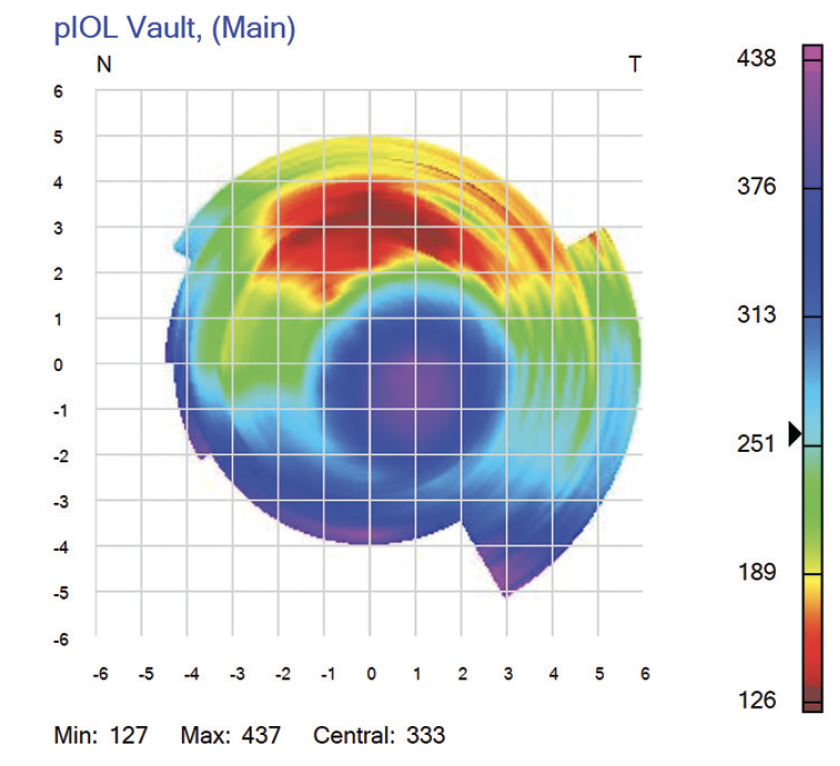
Figure 4. Map showing the vaulting of an Evo Visian ICL in vivo, as captured with the ArcScan Insight 100. The central vault is 370 µm, but the midperipheral vault superiorly is 135 µm.
Figures 3 and 4 courtesy of Dan Z. Reinstein, MD, MA(Cantab), FRCSC, DABO, FRCOphth, FEBO, PGDip CRS, Cert LRS
An important point to consider is that the current situation could have been avoided with a better method of choosing the ICL size. The surgeon used the Orbscan WTW distance, as recommended by the manufacturer’s sizing protocol. The WTW distance was said to be essentially the same for both eyes but resulted in a vaulting difference of 300 µm between eyes. In actuality, the eye with the lower vault had a smaller WTW distance (11.6 mm OS vs 11.7 mm OD). Several studies have demonstrated a weak correlation between external and anterior chamber ocular measurements and the internal anatomy and dimensions of the posterior chamber, even with multivariate analysis.7,15-20 Significant scatter leads to outliers such as this case. It is well established that more accurate vault prediction than WTW sizing can be achieved with sulcus-based measurements using high-frequency handheld ultrasound biomicroscopy15,18 or anterior chamber–based measurements (scleral spur to scleral spur) using OCT.7,21
My colleagues and I developed a sizing formula using the ArcScan Insight 100 that we have been using in our clinic since 2018. The formula is freely available for use at www.iclsizing.com.14 The multivariate model includes parameters never before described for sizing the ICL. The most powerful predictor is the inner diameter of the ciliary body. Also included are the sulcus-to-sulcus lens rise, the scotopic pupillary diameter (0.04 Lux), and the ICL power and size. The formula predicts the vault of each size lens in the Evo Visian family so that surgeons can choose the optimal vault based on the anterior chamber depth and iridocorneal angle. The accuracy of the vault predicted by this model appears to be the highest ever demonstrated. In our preliminary study of 147 eyes, the vault was within ±100, ±200, and ±300 µm of the prediction in 62%, 84%, and 94% of eyes, respectively.14 In a presentation at the ArcScan booth during the 2021 AAO Annual Meeting, Gregory D. Parkhurst, MD, FACS, reported similar findings using our formula. AS-OCT internal anterior chamber dimensions can be expected to be better than WTW distance but cannot be expected to be as good as directly measured posterior chamber sizing. Outliers such as this case occur when the dimensions of the posterior chamber are not accurately predicted by the indirect anterior chamber measurements.
As a final note, another source of error in vault prediction is the implantation technique. Footplates can be left high in the sulcus rather than depressed into the ciliary body or zonular complex. My colleagues and I found that footplate insertion was in the zonules, ciliary body, and sulcus in 48.3%, 49.2%, and 2.5% of cases, respectively.14

WALTER SEKUNDO, MD, PHD
I would have the patient return every 6 months for observation. The vault in the right eye is perfect, but the vault in the left eye raises concern. I have a few patients with a vault that is slightly less than 100 µm who did not develop signs of cataract. From a legal point of view, I would involve the patient in the decision of how to proceed. If the endothelial cell count is satisfactory and stable and if the vault continues to decrease, I recommend an IOL exchange in the left eye. If the vault remains the same and the crystalline lens is clear, I recommend continued observation.
I do not have specific cutoffs for high and low vaults. Particularly with a high vault, other factors such as anterior chamber depth, the angle, and iris color (a dark iris predisposes the eye to pigmentary glaucoma in my experience) play a role.
I use the STAAR Surgical calculator based on WTW distance, which I measure with three different devices. I believe that VHFDU is a great asset for calculating ICL size, but the cost of the device is high.

WHAT I DID: SUPHI TANERI, MD, FEBOS-CR
As all of the panelists point out, there is no clear consensus on cutoff values for vaulting or for calculating the ideal ICL size. The patient underwent surgery in 2012, when I did not have access to AS-OCT or VHFDU. I have observed the patient every 6 months for almost 10 years now. Figure 5 shows recent OCT scans. The patient’s uncorrected distance visual acuity is 20/12.5 OU. The refractions are stable at 0.25 -0.50 x 1º = 20/10 OD and +0.25 = 20/12.5 OS. The crystalline lenses are still clear.
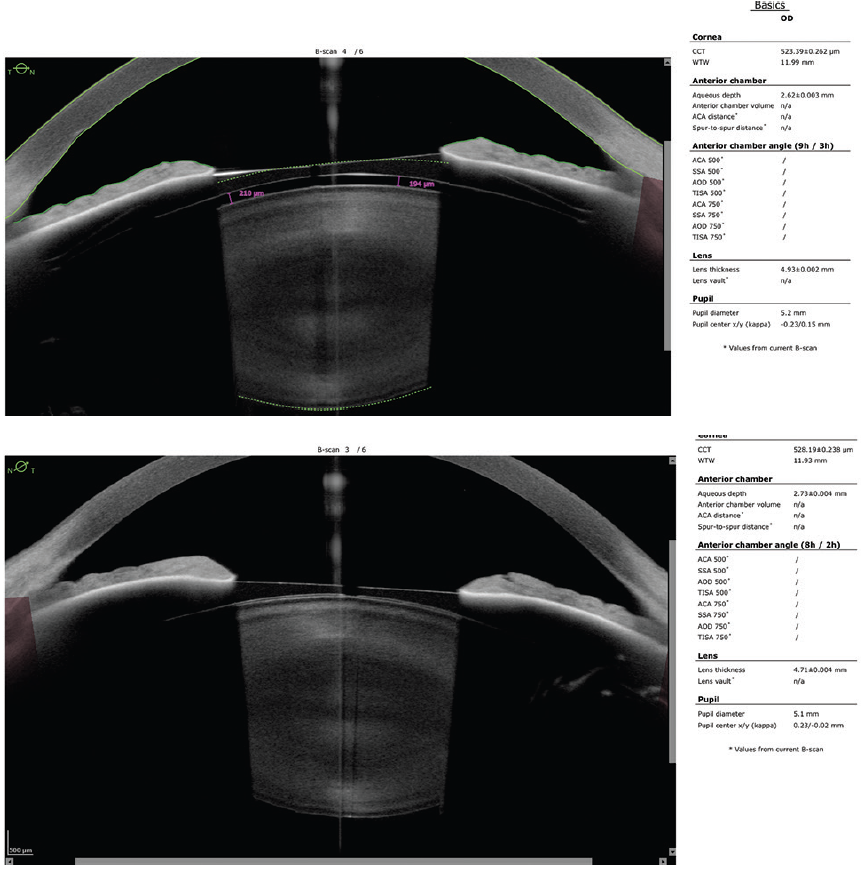
Figure 5. An AS-OCT scan obtained almost 10 years after implantation of the Evo Visian ICL shows a clear crystalline lens and such a low ICL vault that it is no longer detectable.
Figure 5 courtesy of Suphi Taneri, MD, FEBOS-CR
I have 4 to 10 years of follow-up on the 10 eyes in which I implanted an Evo Visian ICL that have a low vault. I have not exchanged any of these lenses to date solely because of a low vault. According to the FDA study, an ICL exchange is also associated with cataract formation. This case and other anecdotal reports suggest that the KS-AquaPort has reduced the incidence of cataract formation, even with a low vault, but long-term follow-up studies are needed to quantify the risk.
1. Reinstein DZ, Vida RS, Archer TJ. Visual outcomes, footplate position and vault achieved with the Visian Implantable Collamer Lens for myopic astigmatism. Clin Ophthalmol. 2021;15:4485-4497.
2. STAAR Surgical announces first Visian ICL V4c implants in Europe. News release. STAAR Surgical; June 27, 2011. https://staar.com/news/2011/staar-surgical-announces-first-visian-icl-v4c-implants-in-europe
3. Alfonso JF, Fernández-Vega L, Lisa C, Fernandes P, González-Meijome J, Montés-Micó R. Long-term evaluation of the central vault after phakic Collamer lens (ICL) implantation using OCT. Graefes Arch Clin Exp Ophthalmol. 2012;250(12):1807-1812.
4. Gonzalez-Lopez F, Bouza-Miguens C, Tejerina V, Mompean B, Ortega-Usobiaga J, Bilbao-Calabuig R. Long-term assessment of crystalline lens transparency in eyes implanted with a central-hole phakic collamer lens developing low postoperative vault. J Cataract Refract Surg. 2021;47(2):204-210.
5. Fernández-Vigo JI, Macarro-Merino A, Fernández-Francos J, et al. Computational study of aqueous humor dynamics assessing the vault and the pupil diameter in two posterior-chamber phakic lenses. Invest Ophthalmol Vis Sci. 2016;57(11):4625-4631.
6. Matarazzo F, Day AC, Fernandez-Vega Cueto L, Maurino V. Vertical implantable collamer lens (ICL) rotation for the management of high vault due to lens oversizing. Int Ophthalmol. 2018;38(6):2689-2692.
7. Nakamura T, Isogai N, Kojima T, Yoshida Y, Sugiyama Y. Implantable collamer lens sizing method based on swept-source anterior segment optical coherence tomography. Am J Ophthalmol. 2018;187:99-107.
8. Igarashi A, Shimizu K, Kato S, Kamiya K. Predictability of the vault after posterior chamber phakic intraocular lens implantation using anterior segment optical coherence tomography. J Cataract Refract Surg. 2019;45(8):1099-1104.
9. Trancón AS, Manito SC, Sierra OT, Baptista AM, Serra PM. Determining vault size in implantable collamer lenses: preoperative anatomy and lens parameters. J Cataract Refract Surg. 2020;46(5):728-736.
10. Choi KH, Chung SE, Chung TY, Chung ES. Ultrasound biomicroscopy for determining visian implantable contact lens length in phakic IOL implantation. J Refract Surg. 2007;23(4):362-367.
11. Yu Y, Zhang C, Zhu Y. Femtosecond laser assisted cataract surgery in a cataract patient with a “0 vaulted” ICL: a case report. BMC Ophthalmol. 2020;20(1):179.
12. Kato S, Shimizu K, Igarashi A. Vault changes caused by light-induced pupil constriction and accommodation in eyes with an implantable collamer lens. Cornea. 2019;38(2):217-220.
13. Chen X, Miao H, Naidu RK, Wang X, Zhou X. Comparison of early changes in and factors affecting vault following posterior chamber phakic implantable collamer lens implantation without and with a central hole (ICL V4 and ICL V4c). BMC Ophthalmol. 2016;16(1):161.
14. Reinstein DZ, Archer TJ, Vida RS, Piparia V, Potter J. New sizing parameters and model for predicting postoperative vault for the implantable collamer lens (ICL) posterior chamber phakic intraocular lens. J Refract Surg. Forthcoming 2022.
15. Dougherty PJ, Rivera RP, Schneider D, Lane SS, Brown D, Vukich J. Improving accuracy of phakic intraocular lens sizing using high-frequency ultrasound biomicroscopy. J Cataract Refract Surg. 2011;37(1):13-18.
16. Oh J, Shin HH, Kim JH, Kim HM, Song JS. Direct measurement of the ciliary sulcus diameter by 35-megahertz ultrasound biomicroscopy. Ophthalmology. 2007;114(9):1685-1688.
17. Kim KH, Shin HH, Kim HM, Song JS. Correlation between ciliary sulcus diameter measured by 35 MHz ultrasound biomicroscopy and other ocular measurements. J Cataract Refract Surg. 2008;34(4):632-637.
18. Kojima T, Yokoyama S, Ito M, et al. Optimization of an implantable collamer lens sizing method using high-frequency ultrasound biomicroscopy. Am J Ophthalmol. 2012;153(4):632-637.
19. Reinstein DZ, Archer TJ, Silverman RH, Rondeau MJ, Coleman DJ. Correlation of anterior chamber angle and ciliary sulcus diameters with white-to-white corneal diameter in high myopes using Artemis VHF digital ultrasound. J Refract Surg. 2009;25(2):185-194.
20. Rondeau MJ, Barcsay G, Silverman RH, et al. Very high frequency ultrasound biometry of the anterior and posterior chamber diameter. J Refract Surg. 2004;20(5):454-464.
21. Nakamura T, Isogai N, Kojima T, Yoshida Y, Sugiyama Y. Optimization of implantable collamer lens sizing based on swept-source anterior segment optical coherence tomography. J Cataract Refract Surg. 2020;46(5):742-748.




