

Postoperative opacification of an IOL caused by calcification (Figure 1) is a rare phenomenon typically observed only with hydrophilic IOLs.1,2 IOL opacification occurs when deposits of calcium phosphate accumulate on the surface of the IOL and/or within the lens (see Clinical Presentation).3 Quality of vision is affected to varying degrees with this phenomenon.
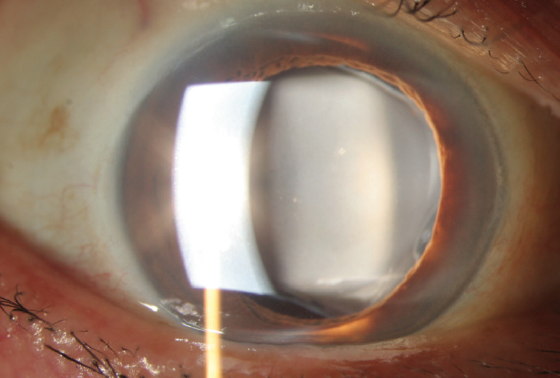
Figure 1. A calcified IOL appears as a cloudy lens.
CLINICAL PRESENTATION
IOL calcification presents as a cloudy lens (Figure 1). A surface irregularity appears in the central part of the optic, or calcium deposits are distributed over different parts of the IOL. The appearance can be similar to posterior capsular opacification.
CLASSIFICATION
A case of IOL calcification was first reported in 2000 and involved a Hydroview hydrogel IOL (Bausch + Lomb).4,5 Multiple reports have since been published that describe the calcification of various IOL surfaces and materials, mostly acrylic hydrophilic IOLs but also silicone, PMMA, and, rarely, hydrophobic IOLs. Neuhann et al identified three forms of IOL calcification: primary, secondary, and false-positive (ie, pseudocalcification).1
Primary calcification. In this form, the complication is inherent to the IOL. The formulation of the polymer was defective, the mode of fabrication of the IOL was problematic, or there was a problem with the packaging process. Calcification occurs in an otherwise healthy eye and is generally not associated with preexisting disease.
During the early 2000s, large series of calcification deposits were reported with four IOLs: the Hydroview,6 Memory Lens (Ciba Vision, later merged with Alcon), SC60B-OUV (Medical Developmental Research), and Aqua-Sense7 (Ophthalmic Innovations International). Either these IOLs were removed from the market or a change was made to their composition or packaging.
Secondary calcification. The IOL itself is not the source of the problem with secondary calcification. Instead, environmental circumstances (eg, changes in the aqueous humour surrounding the implant, disruption of the blood-aqueous barrier by disease6,8-11) cause calcium to be deposited on the surface of the IOL.
Pseudocalcification. Other pathology is misdiagnosed as calcification, perhaps because of tissue artifacts,12 or false-positive staining for calcium occurs after IOL extraction.
PATHOPHYSIOLOGY
The precise factors involved in IOL calcification have yet to be determined. A brief explanation of what is known follows.
Primary calcification. Werner et al found that silicone deposits on the surface of an IOL played a role in the calcification of two hydrophilic acrylic lenses, the MemoryLens and Aqua-Sense.13 Dorey et al showed that silicone can act as a nidus for calcium deposition,14 and Wu et al reported that the interaction between silicone and fatty acids present in the aqueous humour can precipitate the deposition of calcium on the surface of an IOL.15 A few studies have shown that the hydrophobic surface of hydrophilic acrylic IOLs does not protect them from the development of calcification. This is because the phenomenon is initiated in the hydrophilic subsurface.16
Secondary calcification. Multiple factors are causative. The substance (air, gas, silicone oil, tissue plasminogen activator) injected during surgery may come into direct contact with the IOL, or the substance may cause metabolic changes to the aqueous humour that lead to calcification.6 A breakdown of the blood-aqueous barrier caused by surgery or by a disease such as diabetes can exacerbate this phenomenon.
MANAGEMENT
There is currently no medical treatment for IOL calcification. Attempts to clear the capsule with an Nd:YAG laser are not only useless but also dangerous because they increase the risk of vitreous prolapse from the capsulotomy created for IOL extraction.
If IOL calcification causes a severe loss of visual acuity or foggy vision, an IOL exchange is the only option (scan the QR code now to watch a related surgical video). It is, however, associated with a high rate of intraoperative complications such as endothelial cell loss, corneal decompensation, astigmatism induced by enlargement of the incision, and a potential inability to place the replacement IOL in the capsular bag. Informed consent entails educating patients about these risks. Discussion should address alternatives such as implanting the replacement IOL in the ciliary sulcus as well as iris and transscleral fixation. The conversation should also address the balance between the risks of vitreous prolapse, retinal tears, and retinal detachment7 with the benefits of surgery.
It is important to ensure that patients have realistic refractive expectations before surgical intervention, particularly if the IOL to be explanted is designed to correct astigmatism and/or presbyopia.17 The patient’s lifestyle should be taken into consideration when choosing the power and design of the replacement IOL. It is preferable to implant a hydrophobic lens in an effort to prevent a recurrence of calcification.
Before surgery, preoperative analysis of the cornea (eg, endothelial cell count) and retina (eg, OCT imaging of the macula) is mandatory. Intraoperatively, copious amounts of a dispersive OVD should be used to protect the corneal endothelium and open the bag. To remove the IOL, the optic is lifted above the capsulorhexis, and the haptics are then flexed out. If the haptics are attached to the periphery of the bag, they may be amputated and left in place and only the optical center of the IOL extracted. The IOL may be removed by first cutting it into two or three pieces or by the use of a Pac-Man technique (Figure 2).
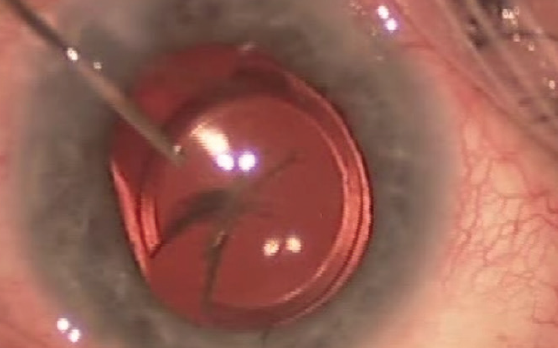
Figure 2. The IOL is explanted by cutting the optic into a shape that resembles Pac-Man from the popular maze arcade game.
It is always preferable to implant the replacement IOL in the capsular bag. If that is not possible, implantation in the ciliary sulcus is the second-best option when anterior capsular support is adequate. Otherwise, iris and scleral fixation are the only remaining options. (For more on scleral fixation, see “Yamane Tips and Tricks,”.)
CONCLUSION
Patients who undergo an IOL exchange to address late calcification require long-term observation to detect calcification of the replacement IOL if it occurs. It is also necessary to monitor the IOL in the contralateral eye, especially if an IOL of the same type and model as the explanted lens was used.
1. Neuhann IM, Kleinmann G, Apple DJ. A new classification of calcification of intraocular lenses. Ophthalmology. 2008;115(1):73-79.
2. Grzybowski A, Markeviciute A, Zemaitiene R. A narrative review of intraocular lens opacifications: update 2020. Ann Transl Med. 2020;8:22.
3. Kanclerz P, Yildirim TM, Khoramnia R. Microscopic characteristics of late intraocular lens opacifications. Published October 22, 2020. Arch Pathol Lab Med. doi: 10.5858/arpa.2019-0626-RA
4. Fernando GT, Crayford BB. Visually significant calcification of hydrogel intraocular lenses necessitating explantation. Clin Experiment Ophthalmol. 2000;28(4):280-286.
5. Werner L, Apple DJ, Escobar-Gomez M, et al. Postoperative deposition of calcium on the surfaces of a hydrogel intraocular lens. Ophthalmology. 2000;107(12):2179-2185.
6. Darcy K, Apel A, Donaldson M, et al. Calcification of hydrophilic acrylic intraocular lenses following secondary surgical procedures in the anterior and posterior segments. Br J Ophthalmol. 2019;103(12):1700-1703.
7. Dagres E, Khan MA, Kyle GM, Clark D. Perioperative complications of intraocular lens exchange in patients with opacified Aqua-Sense lenses. J Cataract Refract Surg. 2004;30(12):2569-2573.
8. Mhéalóid ÁN, Fulcher T, O’Keefe M. Anterior surface opacification of intraocular lenses after Descemet’s stripping automated endothelial keratoplasty. Case Rep. 2015;2015:bcr2015213216.
9. Mojzis P, Studeny P, Werner L, Piñero DP. Opacification of a hydrophilic acrylic intraocular lens with a hydrophobic surface after air injection in Descemet-stripping automated endothelial keratoplasty in a patient with Fuchs dystrophy. J Cataract Refract Surg. 2016;42(3):485-488.
10. Schrittenlocher S, Penier M, Schaub F, Bock F, Cursiefen C, Bachmann B. Intraocular lens calcifications after (triple-) Descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2017;179:129-136.
11. Khurana RN, Werner L. Calcification of a hydrophilic acrylic intraocular lens after pars plana vitrectomy. Retin Cases Brief Rep. 2018;12(3):204-206.
12. Werner L, Wallace JK, Balendiran V, Shumway C, Ellis N, Mamalis N. Surface deposits mimicking calcification on a hydrophobic acrylic intraocular lens. J Cataract Refract Surg. 2019;45(7):1036-1039.
13. Werner L, Hunter B, Stevens S, Chew JJ, Mamalis N. Role of silicon contamination on calcification of hydrophilic acrylic intraocular lenses. Am J Ophthalmol. 2006;141(1):35-43.
14. Dorey MW, Brownstein S, Hill VE, et al. Proposed pathogenesis for the delayed postoperative opacification of the Hydroview hydrogel intraocular lens. Am J Ophthalmol. 2003;135(5):591-598.
15. Wu W, Guan X, Tang R, et al. Calcification of intraocular implant lens surfaces. Langmuir. 2004;20(4):1356-1361.
16. Bang SP, Moon K, Lee J-H, Jun JH, Joo C-K. Subsurface calcification of hydrophilic refractive multifocal intraocular lenses with a hydrophobic surface: a case series. Medicine (Baltimore). 2019;98;50.
17. Moschos MM, Laios K, Lavaris A, et al. Refractive nightmares revisited: calcification of a multifocal intraocular lens. In Vivo. 2018;32(5):1265-1267.




