CASE PRESENTATION
A 59-year-old woman underwent bilateral radial keratotomy (RK) nearly 30 years ago. The patient developed herpes zoster virus (HZV) keratitis in her left eye in 2015, and she has been treated with topical prednisolone acetate 1% suspension since that time. More recently, she developed a small infiltrate (Figure 1) deep within one of the RK incisions (Figure 2), with superficial neovascularization (Figure 3) and feathery extensions into the deep stroma (Figure 4). This lesion has altered corneal topography (Figure 5). Confocal microscopy (Figure 6) confirms crystals rather than filamentous fungi.

Figure 1. A small infiltrate involving an RK incision in the midperipheral cornea.

Figure 2. Advanced imaging with the Spectralis (Heidelberg Engineering) shows deep stromal involvement of the infiltrate.

Figure 3. Superficial neovascularization (arrow) along the RK incision.

Figure 4. Feathery extensions of the infiltrate deep into the corneal stroma.
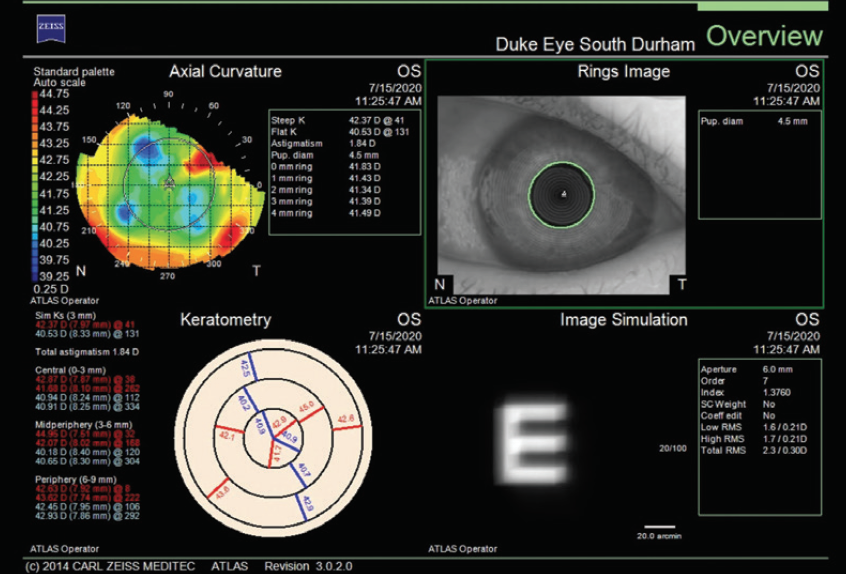
Figure 5. The infiltrate is affecting corneal topography (Atlas 9000, Carl Zeiss Meditec).

Figure 6. Confocal microscopy supports a diagnosis of ICK.
Figures 1–4 courtesy of Cristiaan Lopez-Miro, ophthalmic photographer, Duke Eye Center; Figures 5 and 6 courtesy of Alan N. Carlson, MD
With a presumed diagnosis of infectious crystalline keratopathy (ICK) deep within the RK incision, how would you proceed?
—Case prepared by Alan N. Carlson, MD

DANIEL G. DAWSON, MD
From the clinical scenario presented, this patient appears to have four incisions from an RK procedure performed 30 years ago and a new white infiltrate without an overlying epithelial defect but with an associated, adjacent single frond of intrastromal neovascularization at the superotemporal RK scar. The most common causes of interstitial keratitis (IK) in the United States are herpes simplex and zoster viruses and syphilis infections (> 50% of cases of IK in the United States are caused by herpes viruses and syphilis spirochete bacteria1). Other possible etiologies include atypical bacterial diseases (Lyme disease, tuberculosis, leprosy, brucellosis, and leptospirosis), other viral diseases (Epstein-Barr, human T-cell leukemia virus type 1, mumps, and measles viruses), parasitic disease (onchocerciasis, trypanosomiasis, microsporidiosis, and Acanthamoeba keratitis), and immune-mediated disease (Cogan syndrome, sarcoidosis, mycosis fungoides, and contact lens–associated neovascularization).
Alternatively, this could be a case of true ICK with neovascularization secondary to the infiltrate. ICK, however, is a rare disorder that occurs most commonly after penetrating keratoplasty with the long-term placement of sutures and the prolonged use of corticosteroid therapy.
Based on the infiltrate’s appearance on confocal microscopy (resolution is 2–3 µm, and each piece of crystalline-like material appears to be approximately 15–20 x 200–300 µm in size), the white infiltrate is consistent with lipid that leaked from the adjacent IK stromal blood vessel. Streptococcal bacteria (0.5–2.0 µm in size) found in ICK cases are much smaller organisms that would be seen only as intralamellar pockets of inconspicuous, smudgy material (ie, too small to resolve on confocal microscopy) rather than the large extracellular structures shown.
On anterior segment OCT, the feathery, crystalline-like lipid material appears to be located deep in the corneal stroma. The workup should include a rapid plasma reagin or venereal disease research laboratory test and a fluorescent treponemal antibody absorption test or microhemagglutination assay for treponema pallidum antibodies to rule out syphilis.
Topical corticosteroids (being used to control HZV stromal keratitis here) are the mainstay of therapy for the most common forms of IK because these agents control corneal inflammation and are effective both for alleviating acute symptoms of pain, discomfort, and blurred vision and for reducing scarring and neovascularization. The dosing of prednisolone acetate should therefore be increased to four to eight times daily, and the infiltrate should be assessed weekly for changes. For HZV, the adjunctive use of systemic antivirals (oral acyclovir 800 mg twice daily) at maintenance dose levels is recommended to prevent the recurrence of ocular and orofacial HZV.
If the infiltrate worsens after corticosteroid dosing increases, a diagnosis of ICK must be confirmed with culture results (eg, a braided polyglactin suture passed through the infiltrate for culture plating or opening of the RK wound and a direct culture of the infiltrate). Therapy with a combination of broad-spectrum antibacterial agents (eg, fortified vancomycin and topical moxifloxacin) may be initiated while culture results are pending. Unfortunately, with ICK, the organism is usually a Streptococcus species that makes biofilms. It is therefore difficult to resolve the infection with topical therapy alone, and excision that includes the adjacent corneal tissue is required.2
If the infiltrate improves with corticosteroid therapy alone or if ICK resolves 6 months after the initiation of treatment, one could continue the medical therapy long-term or try mitomycin intravascular chemoembolization. In the latter recently developed method, mitomycin C 0.4 mg/mL is delivered via a 33- or 34-gauge needle on a 1-mL syringe into the lumen of the largest abnormal corneal vessel in the peripheral cornea to permanently destroy or blanch the neovascularization in an effort to save the eye.3

MATIAS SOIFER, MD
ICK typically develops when corneal inflammatory responses are attenuated, leading to microbial colonization, commonly with Gram-positive bacteria. This presents as a sequestration of organisms within the stroma resembling crystals owing to their growth within the interlamellar spaces.
This patient presents with various risk factors for developing ICK. RK incisions are known periodically to extrude incisional epithelium, providing access for organism inoculation. Additionally, the corneal immune response is blunted by neurotrophism, resulting from the earlier RK surgery in combination with the herpes zoster ophthalmicus. This impaired neural sensory response, in addition to long-term use of a topical corticosteroid, creates an ideal local immunosuppressive scenario for a slow-growing organism to replicate without eliciting a typical inflammatory response.
The gold standard for diagnostic identification of the offending organism is Gram stain and culture of the lesion. Noninvasive in vivo confocal microscopy (IVCM) is, however, a valuable diagnostic tool for rapidly ruling out the presence of atypical microbial pathogens that can be associated with ICK such as Acanthamoeba and fungi.4 IVCM of this patient reveals multiple hyperreflective, needle-like extensions consistent with ICK, which can be confused with a filamentous fungal keratitis (Figure 7). The use of IVCM allows prompt and more reliable empirical treatment directed toward the most common etiologic organisms—Gram-positive bacteria—while culture results are pending. Because the lesion is not superficial, the RK incision may be cautiously separated, and the infection may be sampled with a small needle or a Kimura spatula. Corneal biopsy is seldom indicated but may be considered if the clinical course unexpectedly worsens.
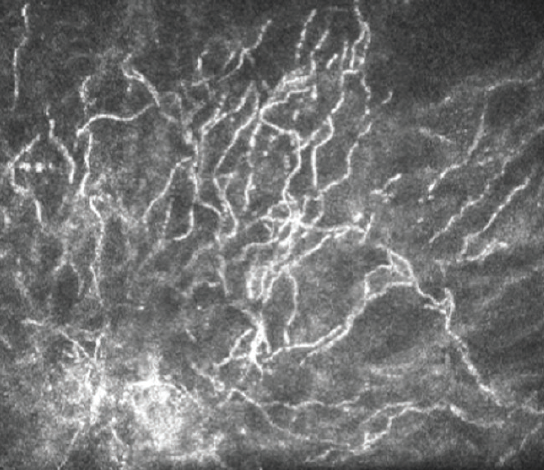
Figure 7. In vivo confocal microscopy of this patient reveals multiple hyperreflective, needle-like extensions consistent with ICK.
Courtesy of Matias Soifer, MD
The first line of empirical treatment against Gram-positive bacteria is topical fortified antibiotics such as vancomycin 50 mg/mL. The clinical response to topical antibiotics is typically gradual. The topical steroid drops should be tapered to enhance an immunologic response to the colonization. The clinical setting guides the dosing and timing of these medications. For example, a recent history of penetrating keratoplasty requires a slower corticosteroid taper than in this case. In refractory cases, intrastromal delivery provides a higher concentration of antibiotics directly to the site of infection and physically disrupts a biofilm microenvironment.5 Success has been reported with intrastromal vancomycin, moxifloxacin, and cefuroxime.4 Finally, recalcitrant progression may warrant treatment with an Nd:YAG laser, which seems to be effective at disrupting the biofilm.6

WHAT I DID: ALAN N. CARLSON, MD
Confocal microscopy is the most significant advance in recent years for the early diagnosis of filamentous fungi, Acanthamoeba, and, in this case, ICK. Fortunately, this patient responded to a course of topical vancomycin 25 mg/mL ophthalmic drops, and resolution of the infectious projections was evident at the slit lamp and with confocal microscopy. The corneal neovascularization remains, and it is currently causing deep lipid keratopathy (Figure 8). I have therefore instructed the patient to administer a low-dose topical corticosteroid (fluorometholone 0.25% ophthalmic suspension) twice daily and to return every few months so that I may monitor her progress.
1. Feldman BH, Tu E, Bunya V, Rose L, Karakus S. Interstitial keratitis. American Academy of Ophthalmology EyeWiki. February 28, 2021. Accessed April 7, 2021. https://eyewiki.org/Interstitial_Keratitis
2. Bunya V, Maki S, Moon C, Ortiz-Morales G. Infectious crystalline keratopathy. American Academy of Ophthalmology EyeWiki. November 5, 2020. Accessed April 7, 2021. https://eyewiki.org/Infectious_Crystalline_Keratopathy
3. Hall MN, Moshirfar M, Amin-Javaheri A, Ouano DP, Ronquillo Y, Hoopes PC. Lipid keratopathy: a review of pathophysiology, differential diagnosis, and management. Ophthalmol Ther. 2020;9(4):833-852.
4. Porter AJ, Lee GA, Jun AS. Infectious crystalline keratopathy. Surv Ophthalmol. 2018;63(4):480-499.
5. Khan IJ, Hamada S, Rauz S. Infectious crystalline keratopathy treated with intrastromal antibiotics. Cornea. 2010;29(10):1186-1188.
6. Masselos K, Tsang HH, Ooi JL, Sharma NS, Coroneo MT. Laser corneal biofilm disruption for infectious crystalline keratopathy. Clin Exp Ophthalmol. 2009;37(2):177-180.




