
More than a billion individuals worldwide experience presbyopia.1 Interest in the surgical correction of the condition remains high, but nonsurgical methods also hold appeal. Current pharmacologic efforts to address presbyopia center on achieving miosis to increase the depth of focus. In 2023, Novartis terminated its US FDA trial of an alternative approach that used a lipoic acid choline ester (UNR844) to soften the crystalline lens when the study’s primary objective was not met.2
The relationship between depth of focus and pupil size is well known.3 Achieving a pupil smaller than 2 mm is necessary to increase depth of focus and near visual acuity significantly (Figure 1).4 The cholinergic activity of miotics stimulates the pupillary sphincter, but most of these agents also stimulate the ciliary muscle, which can cause an accommodative spasm, brow ache, and a myopic shift. The last of these can improve the patient’s near visual acuity but typically degrades their distance visual acuity except when the individual has low hyperopia. Because the concentration of any given cholinergic agent can usually be raised sufficiently to obtain a small pupil, the challenge is not achieving miosis but doing so without concomitantly stimulating the ciliary muscle. What is needed is what I refer to as a pupil-selective miotic.

Figure 1. The relationship between pupil diameter and total depth of focus (A) and the improvement in near vision as a function of pupil diameter under different lighting conditions (B).2-5
This article discusses the latest developments in pharmacologic treatments for presbyopia.
CURRENT MIOTIC AGENTS
The Table shows the current development and approval status of various miotic agents. Of these, only Vuity (pilocarpine HCL ophthalmic solution 1.25%, AbbVie) has been approved by the US FDA and made commercially available. Qlosi (pilocarpine HCL ophthalmic solution 0.4%, Orasis Pharmaceuticals) has also been approved by the US FDA but is not yet available.
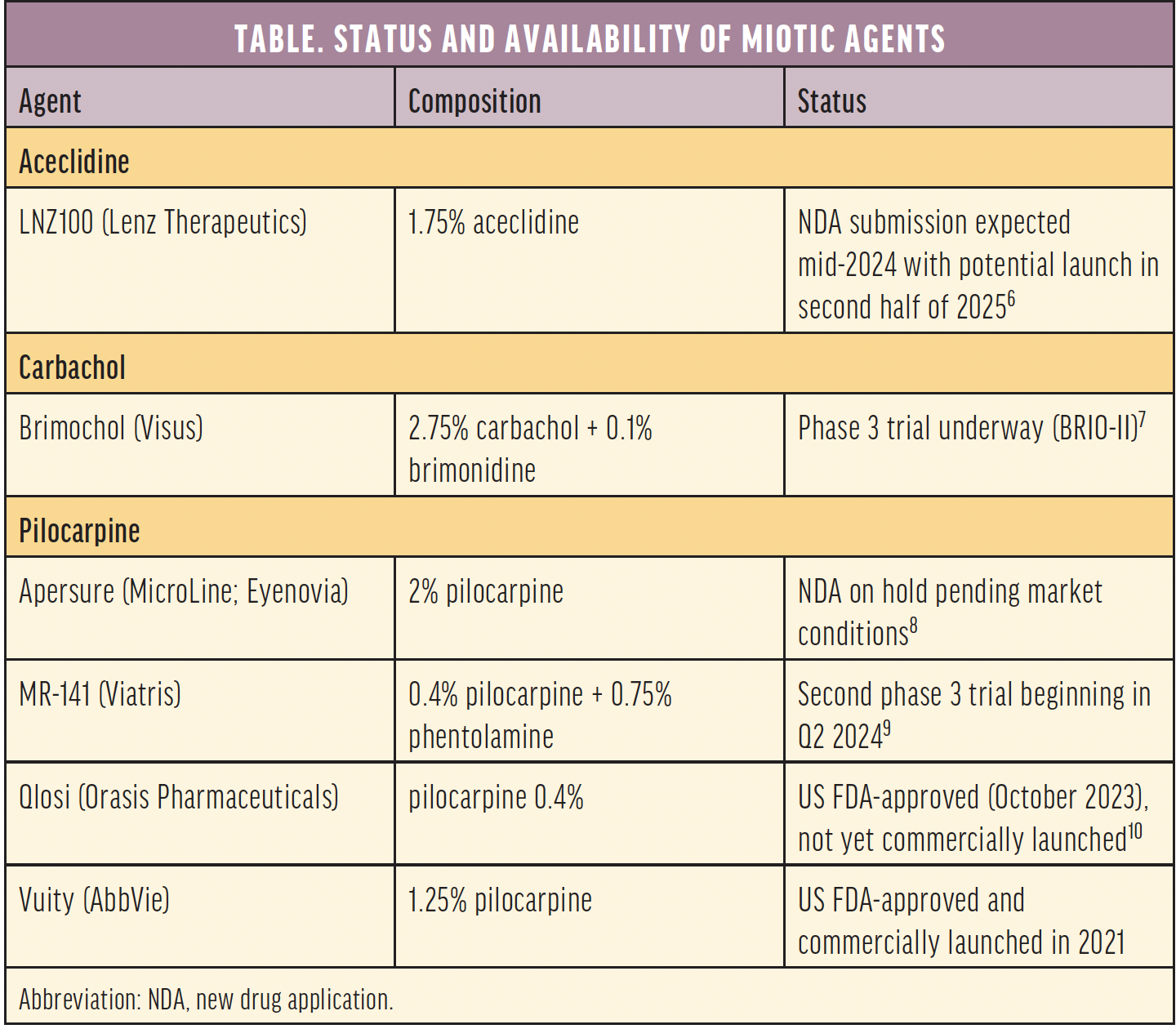
Four of the miotic agents are principally pilocarpine in various proprietary formulations and concentrations: Vuity, Qlosi, Microline (Eyenovia), and Nyxol + Pilocarpine (Viatris). Nyxol contains phentolamine, a nonselective alpha-adrenergic antagonist. Brimochol PF (Visus Therapeutics) is a combination of carbachol and brimonidine. LNZ100 (Lenz Therapeutics) contains the compound aceclidine. Lenz also tested a second compound, LNZ101, which contained aceclidine and brimonidine but chose not to pursue its development because it was not found to be superior to aceclidine alone.
As the first topical treatment for presbyopia, Vuity’s launch in 2021 generated interest and excitement in the ophthalmic and lay press. Although the agent does not achieve a sub-2-mm pupil, the drug improves near visual acuity in many individuals. The pupillary nadir for Vuity occurs 1 hour after instillation. By 4 hours, the mean pupil size has enlarged to approximately 3 mm, providing minimal near benefit. In March 2023, the US FDA approved the drug for twice-daily dosing, extending its duration of effect.
All other compounds containing pilocarpine have affected pupil size similarly to Vuity (Figure 2). Brimochol PF, a fixed combination of carbachol and brimonidine tartrate, has performed slightly better and nearly achieved a 2-mm pupil at the 1-hour mark. The only compounds that have demonstrated the capability of achieving a pupil smaller than 2 mm at their clinically studied concentrations contain aceclidine. This level of miosis persisted for approximately 10 hours after instillation.

Figure 2. Pupil size over time for various miotic treatments.6,11-14
A PUPIL-SELECTIVE MIOTIC?
Decades ago, aceclidine was used outside the United States to treat glaucoma. The agent’s efficacy for IOP reduction was inferior to that of pilocarpine, but aceclidine created miosis without ciliary muscle stimulation by targeting muscarinic receptors specific to the iris sphincter.
Figure 3 shows the concentrations of aceclidine, pilocarpine, and carbachol required to achieve a 50% maximum muscle response for the iris sphincter and ciliary muscles. As shown in this figure, the concentrations of pilocarpine and carbachol that affect the ciliary muscle undesirably do not differ significantly from those required to achieve miosis. In contrast, aceclidine affects the ciliary muscle only at concentrations 22 to 28 times higher than those required to achieve miosis. In theory, aceclidine should behave as a pupil-selective miotic, whereas pilocarpine and carbachol should not. Aceclidine could therefore be used at a concentration that provides much more miosis than any other compound.

Figure 3. The drug concentrations required to achieve a 50% maximum muscle response (EC50) for the iris sphincter and ciliary muscle for aceclidine, pilocarpine, and carbachol. Research based on 29 pairs of eyes and donors ranging from 41 to 89 years of age.15
CLINICAL TRIAL RESULTS
Lenz Therapeutics recently released data from the phase 3 CLARITY trial, and the results were encouraging.6 At 30 minutes and 3 hours after drug instillation, 71% of participants experienced at least 3 lines of improvement in their near visual acuity, and the effect persisted for 10 full hours in 40% of participants. A placebo-corrected headache incidence of 7.6% was reported, and the side effect was mild in most cases.
Peripheral retinal traction is a concern with any miotic agent, but a pupil-selective agent should minimize this risk. The CLARITY 1, 2, and 3 trials enrolled a total of 1,059 participants. LNZ100 was well tolerated, and no serious treatment-related adverse events were observed in the more than 30,000 treatment days across all three CLARITY trials.
LOOKING AHEAD
The public is interested in a safe and effective topical treatment for presbyopia, and the market is large and likely growing. Assuming LNZ100 achieves US FDA approval, what remains to be seen is whether it performs better in the marketplace than the weaker agents currently approved.
All miotic compounds rely on small-aperture optics to achieve their effect. None of them addresses the underlying changes in lens elasticity that cause presbyopia. Efforts in that direction have been unsuccessful thus far, but continued research and development in this area seems worthwhile.
1. Donaldson KE. The economic impact of presbyopia. J Refract Surg. 2021;37(S1):S17-S19.
2. ClinicalTrials.gov. A dose-ranging study to evaluate the safety and efficacy of UNR844 in subjects with presbyopia (READER). ClinicalTrials.gov identifier NCT04806503. Updated August 30, 2023. Accessed May 31, 2024. https://clinicaltrials.gov/study/NCT04806503
3. Ciuffreda K. Accommodation, the pupil, and presbyopia. In: Benjamin WJ, ed. Borish’s Clinical Refraction. 2nd ed. Butterworth-Heinemann Elsevier; 2006:93-144.
4. Charman WN. Pinholes and presbyopia: Solution or sideshow? Ophthalmic Physiol Opt. 2019;39(1):1-10.
5. Xu R, Bradley A, Thibos LN. Effect of target luminance on optimum pupil diameter for presbyopic eyes. Ophthalmic Physiol Opt. 2019;39(5):426-433.
6. Lenz Therapeutics reports first quarter 2024 financial results and provides business updates. Lenz Therapeutics. May 08, 2024. Accessed May 31, 2024. https://ir.lenz-tx.com/news-events/press-releases/detail/13/lenz-therapeutics-reports-first-quarter-2024-financial-results-and-provides-business-updates
7. BioSpace. Visus Therapeutics presents topline clinical data from phase 3 pivotal BRIO-I trial of Brimochol™ PF for the treatment of presbyopia at Eyecelerator @ ASCRS 2023. May 2023. Accessed June 1, 2024. https://www.biospace.com/article/releases/visus-therapeutics-presents-topline-clinical-data-from-phase-3-pivotal-brio-i-trial-of-brimochol-pf-for-the-treatment-of-presbyopia-at-eyecelerator-ascrs-2023
8. Eyenovia. We are the Optejet company. March 2024. Accessed May 31, 2024. https://ir.eyenovia.com/static-files/7f814a97-4eee-46dc-ba28-8d4d4b892696
9. Viatris. R & D Event. March 27, 2024. Accessed May 31, 2024. https://investor.viatris.com/static-files/b01a07f3-e549-4522-8480-c31b359a6a4f
10. Orasis Pharmaceuticals announces FDA approval of Qlosi™ (pilocarpine hydrochloride ophthalmic solution) 0.4% for the treatment of presbyopia. Orasis Pharmaceuticals. October 18, 2023. Accessed June 1, 2024. https://www.orasis-pharma.com/orasis-pharmaceuticals-announces-fda-approval-of-qlosi-pilocarpine-hydrochloride-ophthalmic-solution-0-4-for-the-treatment-of-presbyopia/#:~:text=Ponte%20Vedra%2C%20FL%2C%20October%2018,the%20first%20half%20of%202024
11. Christie WC, Tauber J, Zhang F, Safyan E, Tepelus T, Jerkins G. Gemini I phase 3: AGN-190584 improves intermediate vision in participants with presbyopia. Paper presented at: ASCRS Annual Meeting; July 25, 2021; Las Vegas, NV.
12. Farid M, White DE, Fram NR, Holland EJ. CSF-1 (0.4% pilocarpine HCl ophthalmic solution) for the treatment of presbyopia: NEAR-1 and NEAR-2 phase 3 clinical trials. Paper presented at: ASCRS Annual Meeting; May 6, 2023; San Diego, CA.
13. Ocuphire Pharma, Inc. Ocuphire’s VEGA-1 phase 2 trial in presbyopia meets primary and secondary endpoints. GlobeNewswire. June 30, 2021. Accessed June 2, 2024. http://public.viavid.com/index.php?id=145478
14. Visus Vivid (NCT04774237) phase 2 study, stage 2 presentation. Paper presented at Eyecelerator @ ASCRS Annual Meeting; April 22, 2022; Washington, DC.
15. Ishikawa H, DeSantis L, Patil PN. Selectivity of muscarinic agonists including (+/-)-aceclidine and antimuscarinics on the human intraocular muscles. J Ocul Pharmacol Ther. 1998;14(4):363-373.




