CASE PRESENTATION
A 66-year-old man is referred by his optometrist for an evaluation. A physician by profession, the patient has had difficulty tolerating his glasses since undergoing bilateral cataract surgery combined with Descemet membrane endothelial keratoplasty (DMEK) several months ago. His vision is much better than before surgery, but he feels like his eyes are not working well together.
On examination, the patient’s UCVA is 20/200 OD and 20/50 OS. His BCVA is 20/25 OD with a manifest refraction of +4.25 +1.00 x 040º and 20/20 OS with a manifest refraction of +0.50 +1.50 x 160º. Motility is full, and no afferent pupillary defect is evident. A slit-lamp examination of the right eye finds a clear and fully attached DMEK transplant, a well-positioned posterior chamber IOL (PCIOL), a small amount of vitreous prolapse into the anterior chamber, and an anterior capsular tear inferiorly in the anterior capsulorhexis that extends to the posterior capsule (Figure 1). The DMEK transplant in the left eye is clear, the anterior segment has a normal appearance, and a PCIOL is well positioned.
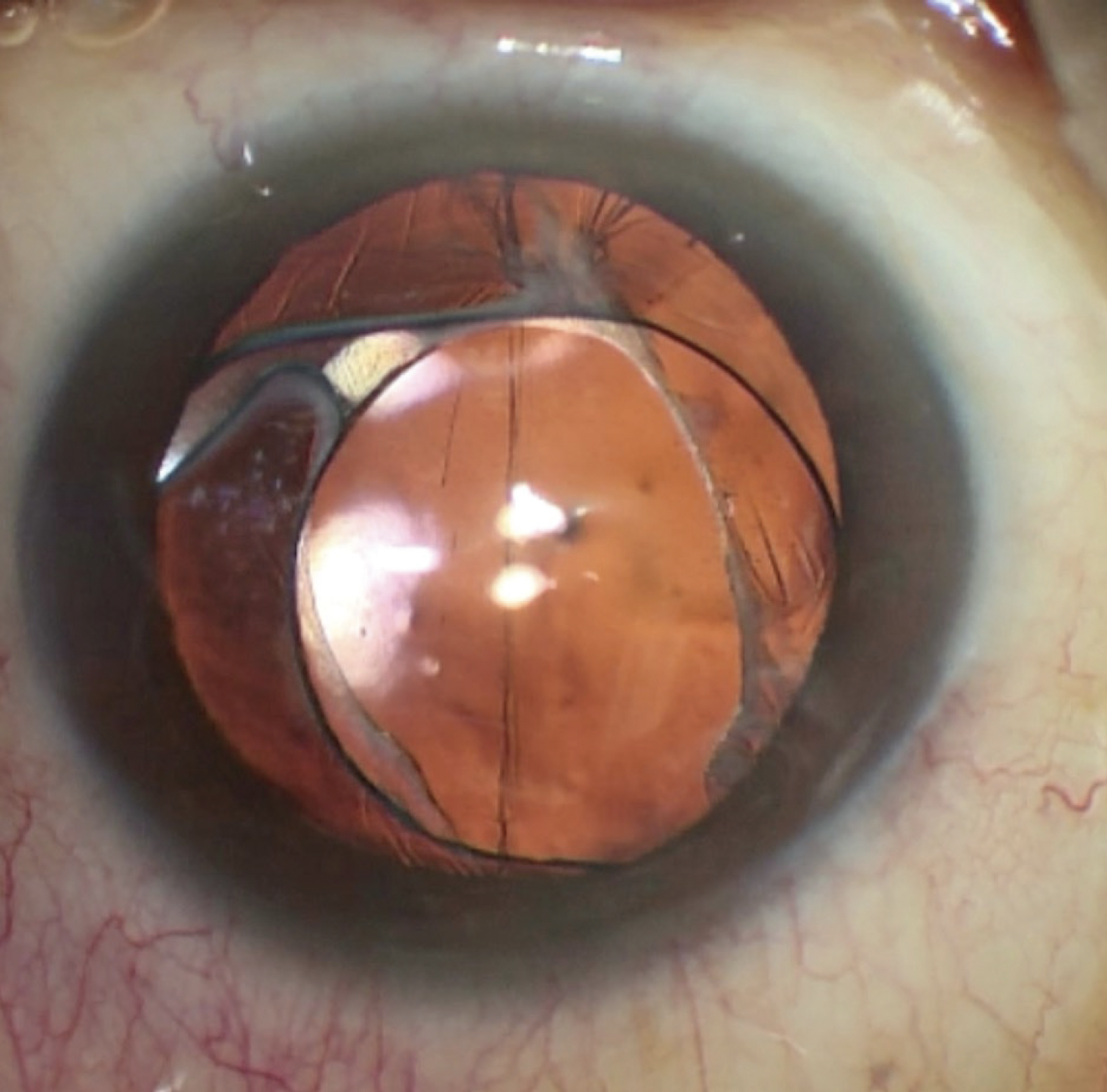
Figure 1. An examination of the right eye reveals a clear cornea (DMEK transplant). The IOL has subluxated slightly but remains within the capsular bag. A large inferior defect in the capsulorhexis extends to the posterior capsule. Vitreous prolapse was noted on the slit-lamp examination but is difficult to see in this image.
A dilated retinal exam finds a normal right eye. The fundus in the left eye has a myopic appearance. No peripheral pathology is observed in either eye.
The patient’s original biometry is shown in Figure 2. The right eye received a +11.50 D one-piece acrylic IOL with a refractive target of -0.50 D. The left eye received a +15.50 D one-piece acrylic IOL with a similar refractive target. A conversation with the original surgeon confirms that no complications were encountered during the combined cataract and DMEK procedure and that the capsular defect was not noted at the time of the surgery. Post–myopic LASIK IOL calculations were performed using the ASCRS website, but significant corneal edema on presentation might have limited the accuracy of keratometry.
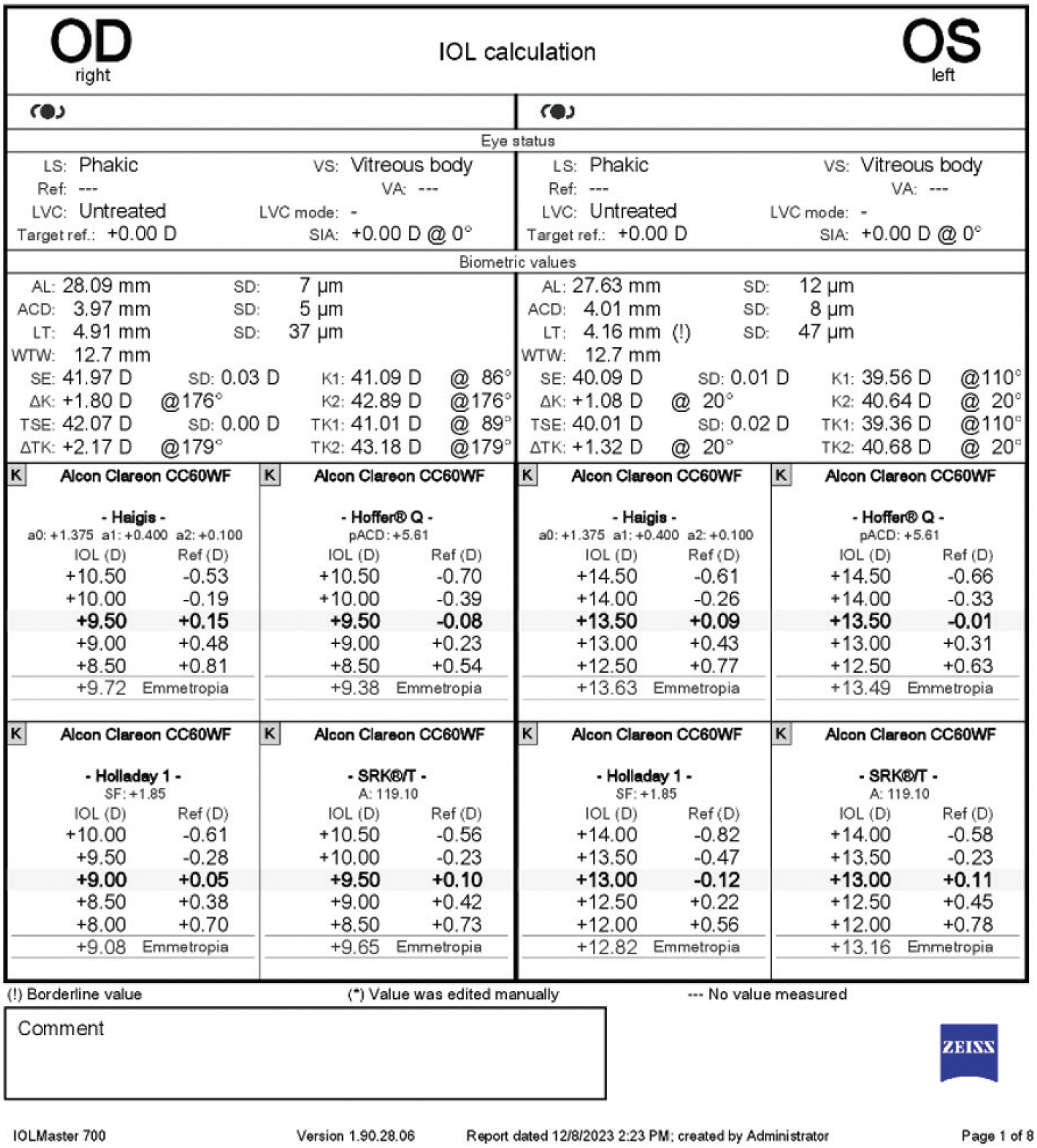
Figure 2. Original biometry used to calculate the IOL power for primary cataract removal and DMEK surgery.
How would you manage the patient’s refractive error? If you would perform an IOL exchange, describe your approach and how you would calculate the new lens power.
— Case prepared by Brandon D. Ayres, MD

ARTHUR B. CUMMINGS, MBCHB, MMED(OPHTH), FCS(SA), FRCSED, CERTLRS, FWCRS
The PCIOL in the right eye is centered adequately, given the complication, and the patient’s corrected distance visual acuity is good. The minimal vitreous prolapse appears to be stable and nonsignificant. Anterior segment OCT or ultrasound biomicroscopy would be performed to confirm bag integrity and IOL position and rule out anterior vaulting or optic tilt.
The patient’s main issue is anisometropia, so management would focus on the least invasive option to restore balance. Given the compromised capsule, I would avoid an IOL exchange. Both a LASIK enhancement and a supplementary sulcus-fixated IOL would be reasonable options.
If vitreous prolapse is likely to interfere with the placement of a supplementary IOL, I would perform an anterior vitrectomy. Otherwise, I would proceed cautiously or defer IOL implantation.
Power selection for a sulcus-based IOL is straightforward; online manufacturer calculators are available, and the required power is approximately 1.5x the spherical equivalent of the manifest refraction. In this case (+4.75 D spherical equivalent), the power of the supplementary IOL would be around +7.00 D.
If an IOL exchange is pursued, approximately +7.00 D would be added to the original +11.50 D IOL, resulting in a power of around +18.50 D. Alternatively, biometry could be repeated and analyzed using the ASCRS post-myopic LASIK or Barrett True K (postrefractive) formulas, with an adjustment for the effective lens position because IOLs implanted in the sulcus sit slightly more anteriorly than those placed in the capsular bag.
If corneal pachymetry permits, a LASIK enhancement would be an option. My preference, however, would be sulcus implantation of a supplementary IOL such as a Sulcoflex (Rayner) or AddOn (1stQ), which would be a safer and more predictable approach.

MICHAEL D. GREENWOOD, MD
The patient’s chief problem is a large hyperopic surprise (approximately +4.00 D) in the right eye that is producing symptomatic anisometropia and binocular dysfunction. The root cause is unreliable keratometry at the time of the original surgery: corneal edema invalidated the assumed anterior-posterior corneal relationship, leading to an underestimation of true corneal power and the implantation of an underpowered IOL.
Critically, the anterior capsular tear with posterior extension and vitreous in the anterior chamber confirm compromise of the capsular bag–zonular complex. Implanting a supplementary IOL in the sulcus would therefore carry the risks of decentration, iris chafe, inflammation, cystoid macular edema, and glaucoma. Laser vision correction would be a poor option because hyperopic ablations (≥ +3.00 D) are unpredictable. An IOL exchange and secondary fixation would be the safest, most durable solution.
After the cornea stabilizes, biometry would be repeated, and the IOL calculation would be performed using total corneal power (eg, Barrett True K or ray tracing) with a target of mild myopia (-0.25 to -0.50 D) for binocular balance. Based on the residual refraction and the original +11.50 D IOL, the replacement lens power would be approximately +15.50 to +16.00 D, individualized to updated measurements. This plan would correct the patient’s refractive error, avoid the pitfalls of sulcus fixation, and preserve the DMEK graft.
Intrascleral haptic fixation of a three-piece PCIOL using the Yamane technique would be performed. To protect the DMEK graft, 27-gauge pars plana vitrectomy trocars would be used to allow infusion and vitrector access posteriorly. An adequate amount of an OVD would be instilled to protect the graft, and the IOL would be removed through the main incision. The replacement IOL would then be implanted and fixated to the sclera via needle docking, after which the haptics would be externalized and flanged.

PRIYA M. MATHEWS, MD, MPH
The patient’s refractive error is causing anisometropia and spectacle intolerance. It would be reasonable to offer him the nonsurgical option of wearing a contact lens in his right eye, which would address the imbalance secondary to anisometropia. Given the subluxated IOL, anterior capsular defect that extends to the posterior capsule, and vitreous prolapse, however, the situation is unstable, and he will likely require surgery in the future.
If the tear had not extended to the posterior capsule, a belt-loop technique could be considered for scleral fixation of the original IOL along with a partial anterior vitrectomy to remove the vitreous in the anterior segment. Because the posterior capsule has been compromised, however, I would recommend an IOL exchange combined with a partial anterior vitrectomy. Biometry and topography would be repeated with the IOLMaster (Carl Zeiss Meditec) and Pentacam (Oculus Optikgeräte), respectively. A CT Lucia (Carl Zeiss Meditec) would be my preferred IOL because the hydrophobic material would prevent lens opacification if the patient needs a repeat DMEK (with SF6 gas) in the future.
Post–myopic LASIK IOL calculations would be performed using the new keratometry values and a refractive target closer to plano. Alternatively, if the patient wishes to minimize his refractive error in the setting of DMEK and astigmatism, intrascleral haptic fixation of a Light Adjustable Lens (RxSight) using the Yamane technique could be considered.

WHAT I DID: BRANDON D. AYRES, MD
After a long, candid discussion with the patient about the risks and benefits of an IOL exchange and the need for an anterior vitrectomy, surgery was scheduled for the right eye. The complexity of IOL power calculations to minimize his need for distance glasses was also explained.
Multiple IOL calculations were performed, including with the Barrett Rx and True K formulas. An average IOL power was then used (Figure 3). A three-piece IOL was selected because placement in the bag was thought likely to be impossible.
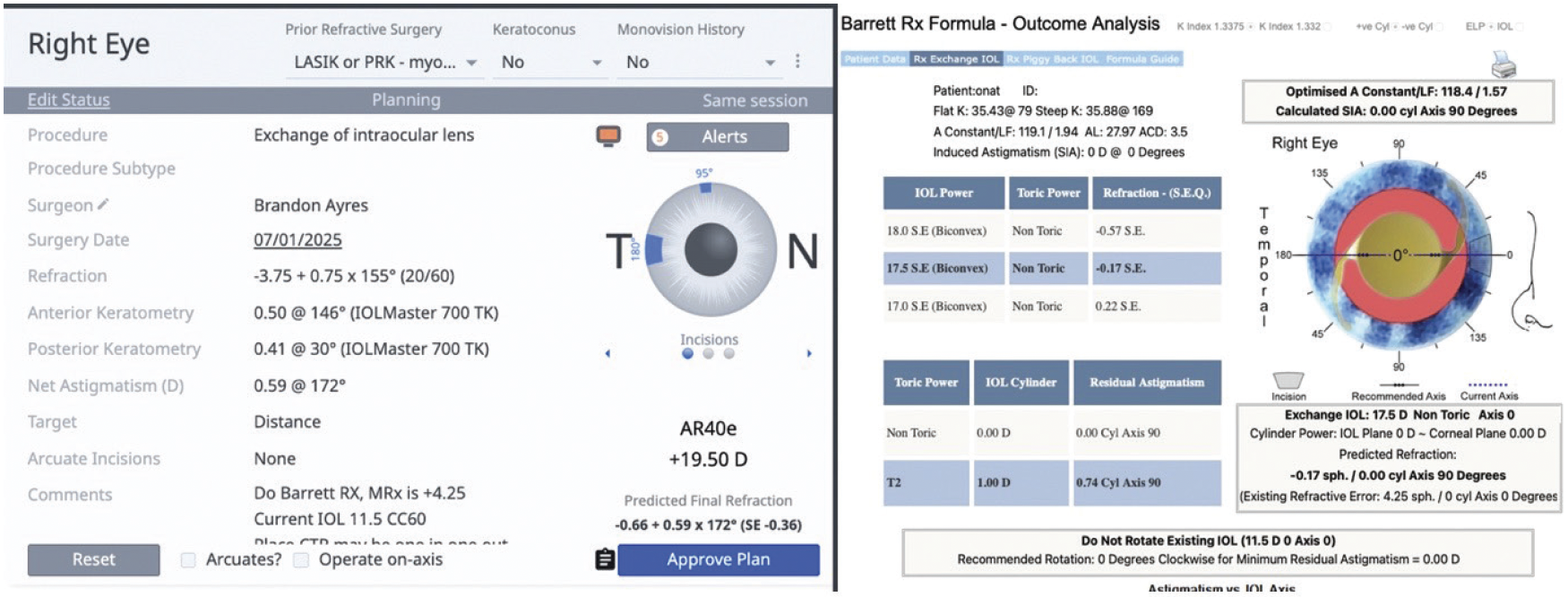
Figure 3. A comparison of IOL calculations for a lens exchange with an emmetropic target. The IOLMaster calculates powers of 19.50 D and 17.50 D with the Barrett True K and Barrett Rx formulas, respectively.
A two-port anterior vitrectomy was performed, and triamcinolone acetonide was instilled to visualize the vitreous that had prolapsed into the anterior chamber (Figure 4). OVD was then instilled to prevent further vitreous prolapse. Next, the original IOL was viscodissected from the capsule (Figure 5) and brought into the anterior chamber, where it was cut into multiple pieces with intraocular scissors. The pieces were then removed through a temporal incision (Figure 6). An additional vitrectomy was subsequently performed to ensure no vitreous was present in the anterior chamber.

Figure 4. A two-port anterior vitrectomy is performed to remove any vitreous from the anterior chamber. Triamcinolone acetonide is instilled to help visualize the vitreous (not shown here).
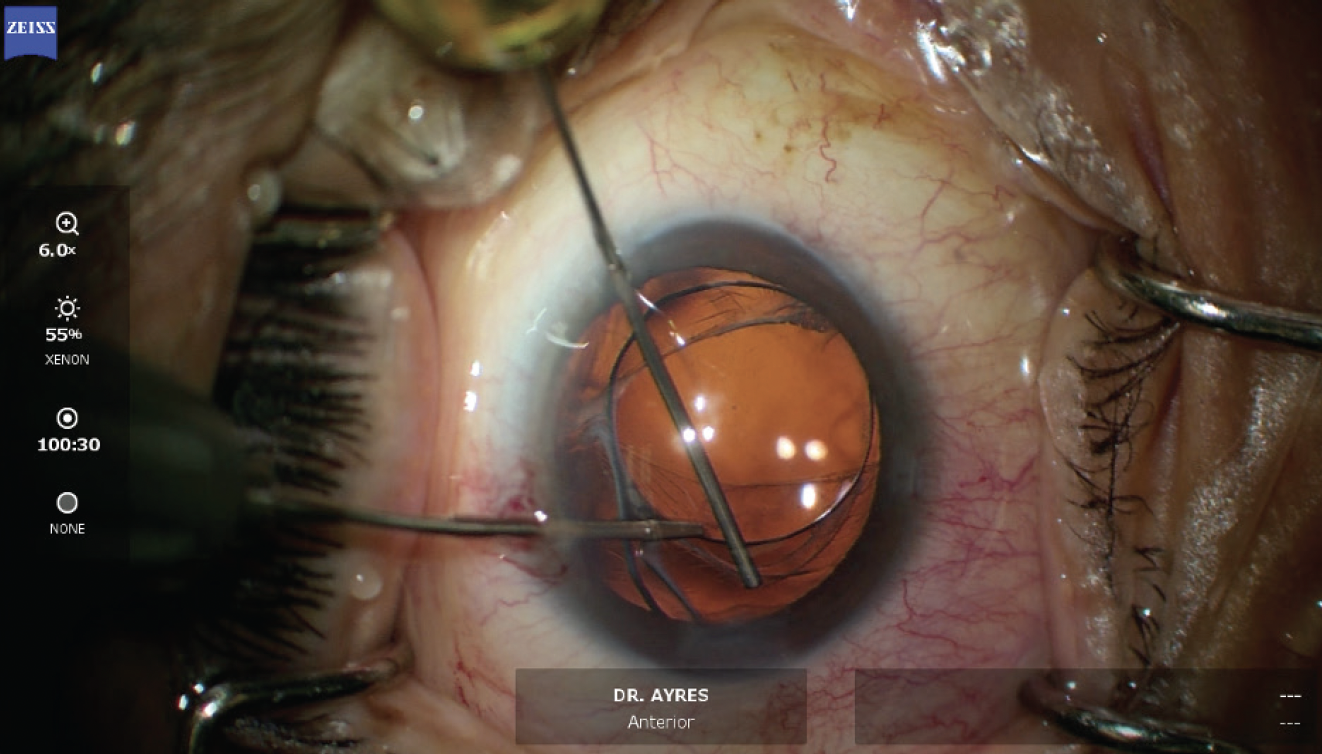
Figure 5. An OVD and microinstruments are used to free the IOL from the capsular bag.
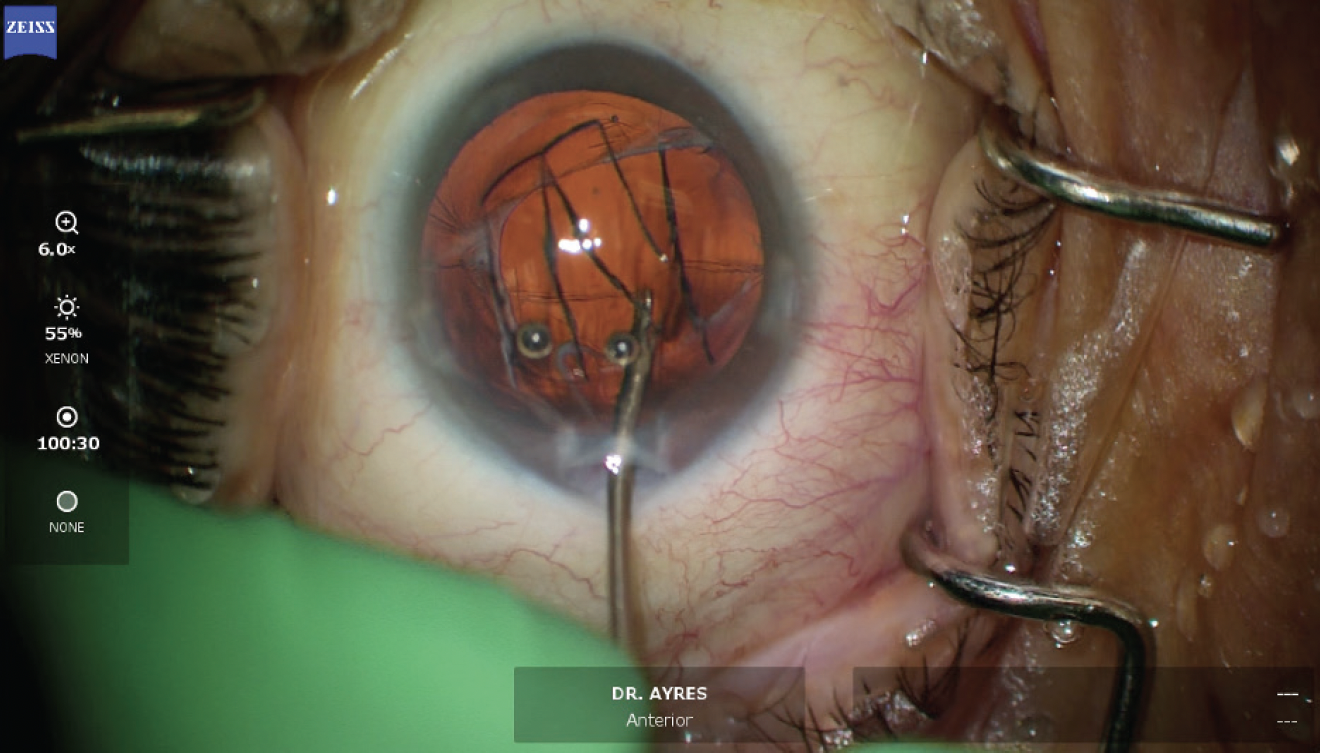
Figure 6. The IOL is cut into small pieces and removed from the anterior chamber.
Upon inspection, an inferior capsular defect was noted to extend from the anterior capsule around to the posterior capsule. This defect prevented the secure placement of an IOL in the capsular bag. The new IOL was carefully implanted in the sulcus (Figure 7). To prevent rotation and eventual dislocation of the lens, a miotic agent was instilled to constrict the pupil, and the lens optic was prolapsed anterior to the iris. The lens haptics were secured to the overlying iris with a 10-0 polypropylene suture (Figure 8). The suture ends were tied using a McCannel technique, and the lens optic was prolapsed through the iris into the posterior chamber. An additional vitrectomy was performed to ensure that no vitreous had prolapsed into the anterior chamber and to remove any remaining OVD. At the conclusion of surgery, the anterior chamber was deep and free of vitreous, the iris was round, and all incisions were watertight (Figure 9).

Figure 7. A three-piece IOL is placed in the sulcus with the haptics resting on the capsular remnants.
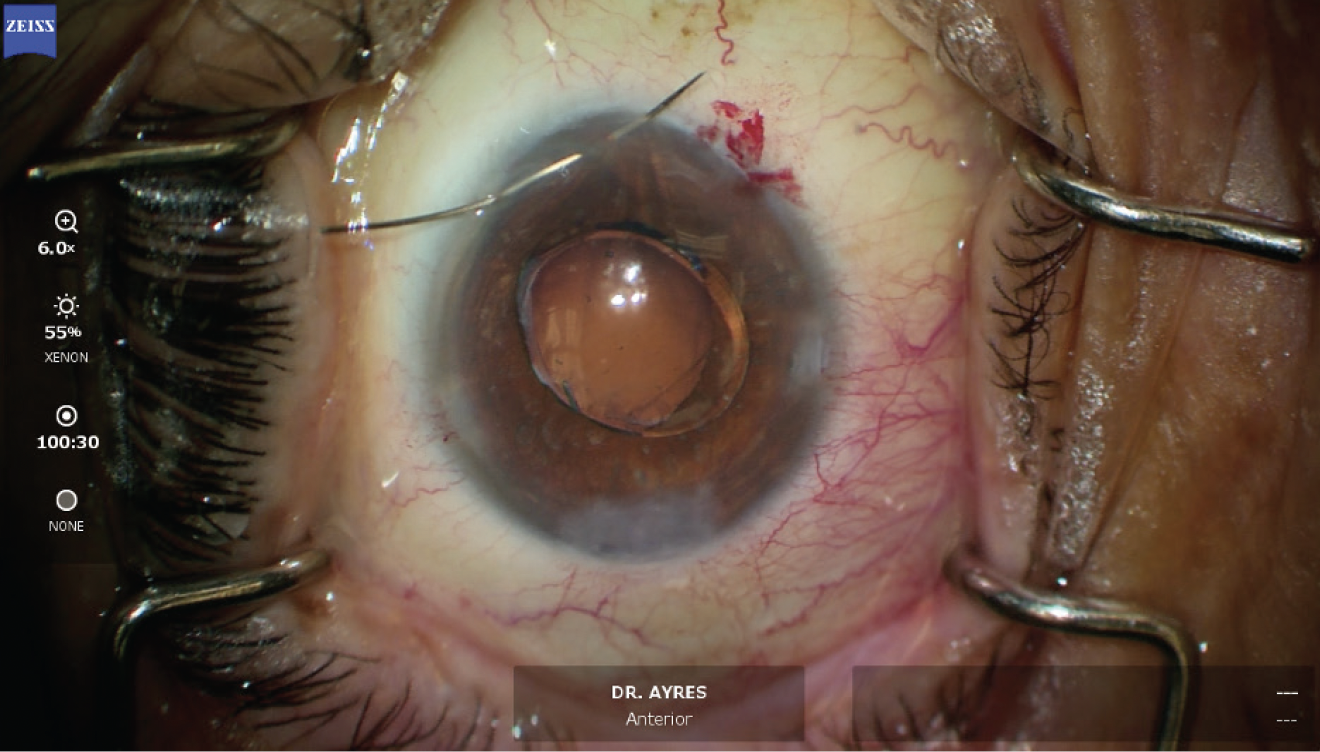
Figure 8. Iris fixation of the haptics using a 10-0 polypropylene suture is performed to prevent IOL rotation and eventual subluxation.

Figure 9. Final appearance of the eye.
One day after surgery, the patient’s UCVA was 20/60 OD. At 1 month, his UCVA was 20/25 OD, and he was highly satisfied.




