

Not only is AI taking over exam lanes, but developers are also eyeing a bigger domain—patients’ homes. AI in ophthalmology has evolved from isolated image-recognition experiments into a multilayered clinical and commercial ecosystem spanning autonomous diagnostics, longitudinal clinical decision support, home monitoring, practice operations, and the early emergence of agentic automation. This progression is driven by accelerating technological innovation, growing social acceptance, and an enabling regulatory framework that defines what AI may do, how it may be deployed, and who ultimately retains clinical authority. If harnessed properly, this development could reshape population-level care for chronic eye disease and improve today’s fragmented, episodic, and often poorly coordinated care model.
REIMBURSEMENT TAILWINDS IN 2025
In 2025, regulatory advances by the US FDA and CMS further supported the AI transition by expanding reimbursement for remote physiologic monitoring (RPM) and principal care management (PCM) services.1
Where the US FDA Draws the Line
The US FDA clarified the boundary between exempt clinical decision support (CDS) software and regulated AI medical devices.2
Beginning with the 2022 CDS Guidance, the agency established that software, including AI, is not regulated when it merely displays medical data and allows the clinician to independently understand the basis of the output but maintain full decision-making authority. Platforms such as Zeiss Forum Compare Reports (Carl Zeiss Meditec) and Harmony (Topcon Healthcare), which aggregate OCT and imaging data without diagnosing progression or issuing treatment recommendations, exemplify this category.
In contrast, the 2025 AI-Enabled Device Software Functions Guidance defines when AI is regulated as a medical device—namely, when it detects disease, diagnoses pathology, classifies clinical states, or autonomously recommends treatment.3
Regulating AI Over Time
The aforementioned framework was reinforced by the Predetermined Change Control Plan Guidance from 2024,4 which governs how US FDA–regulated AI models may evolve after clearance. The Predetermined Change Control Plan specifies which algorithmic changes may occur without resubmission and which require renewed US FDA review. Together, these policies have created an early life cycle regulatory framework for clinical AI.
Reimbursement as the On-Ramp for Autonomous AI
On the payor side, Current Procedural Terminology (CPT) code 92229 (effective January 1, 2021) reimburses providers for point-of-care autonomous AI retinal imaging with automated analysis and report generation, without physician interpretation. This reimbursement milestone helped move autonomous retinal AI from pilot trials to broad deployment in primary care clinics, federally qualified health centers, and pharmacies and allows screening at scale for patients who might otherwise have limited access to eye care.
More recently, Category I CPT codes have expanded reimbursement pathways for RPM and PCM. Beyond creating new economics and incentives for practices, these codes formalize remote care extensions for Medicare beneficiaries and could accelerate the adoption of multidisciplinary, longitudinal care models.
RPM Growth and the 2025 Coding Landscape
Across the world, RPM has shifted from a niche digital health tool to a core component of chronic disease management. A recent mHealth and home-monitoring market analysis estimated that 76.7 million connected home medical monitoring devices were in use in 2023, with volumes projected to reach 140.1 million by 2028 (compound annual growth rate of approximately 12.8%).5 The same analysis projected that RPM revenues would increase from $40.4 billion in 2023 to $77.3 billion by 2028 (compound annual growth rate of approximately 13.9%). This growth is being supported by expanded reimbursement for RPM and PCM services that enable streamlined, remote, multidisciplinary care.
For 2025, RPM codes (CPT 99453–99458) cover digital remote interventions in 20- to 30-minute monthly increments for patients diagnosed with high-risk chronic conditions such as age-related macular degeneration (AMD), diabetic retinopathy (DR), and glaucoma. PCM codes (CPT 99424–99427) address care coordination, patient education, and care management performed by clinic staff or an outsourced service under general oversight.
DR AS THE FIRST PROOF POINT FOR AUTONOMOUS AI
DR provided the first major real-world validation for autonomous AI in ophthalmology. The disease offered an ideal proving ground because screening workflows rely on standardized fundus photography and well-established grading thresholds.
The grading of DR severity is anchored in the Early Treatment Diabetic Retinopathy Study (ETDRS) scale. Key reference points include ETDRS level 35 (more than mild DR [mtmDR]), level 43 (moderate nonproliferative DR), and levels 61 to 71 (proliferative disease with retinal neovascularization).6
Early US FDA–Cleared Autonomous Systems
A comparison of US FDA–cleared autonomous DR systems—including mtmDR sensitivity and specificity and ungradable image rates—is provided in Table 1.7,8
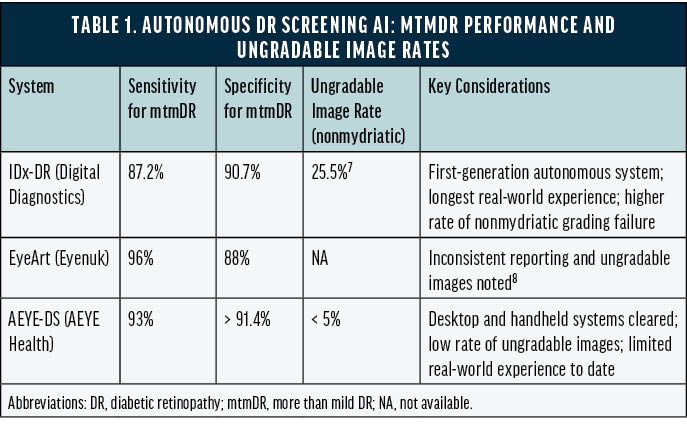
IDx-DR. In 2018, IDx-DR (Digital Diagnostics) became the first US FDA–cleared autonomous AI diagnostic system in any medical specialty. In a pivotal trial, IDx-DR achieved a sensitivity of 87.2% (95% CI, 81.8%–91.2%) and a specificity of 90.7% (95% CI, 88.3%–92.7%) for detecting mtmDR, with an imageability rate of 96.1%. Importantly, the system issued a fully autonomous “refer” decision without physician image interpretation.9
EyeArt. EyeArt (Eyenuk) soon became another US FDA–cleared autonomous system. In real-world screening settings, EyeArt demonstrated 96% sensitivity and 88% specificity for mtmDR and 92% sensitivity with 94% specificity for vision-threatening DR. All eyes with an ETDRS level of 43 or higher were correctly identified as mtmDR positive. Even in primary care clinics, where imaging was often performed by nonophthalmic staff, EyeArt achieved a 97% imageability rate, and 90% of patients received a diagnostic result without pupillary dilation.10
AEYE-DS. In its US FDA clearance study, AEYE-DS (AEYE Health) achieved 93% sensitivity and greater than 91.4% specificity for mtmDR detection using a single image per eye from the TRC-NW400 camera (Topcon), with an imageability rate exceeding 99%.11
From Validation to Reimbursement
The aforementioned technological advances positioned DR as the first disease area to achieve national CMS reimbursement for autonomous AI.
FROM AUTONOMOUS SCREENING TO ASSISTIVE DECISION SUPPORT
Following the autonomous DR wave, AI development shifted toward assistive CDS rather than the replacement of physician judgment. These systems analyze longitudinal and multimodal imaging data, instead of a single snapshot, to support complex disease management in AMD, diabetic macular edema, retinal vein occlusion, and geographic atrophy.
Performance Claims and Current Use Patterns
Strong performance in the areas of OCT-based feature detection and biomarker interpretation has been reported for the following systems:
- RetinAI (EssilorLuxottica) applies longitudinal OCT and biomarker analytics, with reported performance metrics exceeding 94% to 97% for disease feature detection12;
- Altris AI (Altris AMS) has reported cumulative accuracies of approximately 91% for OCT biomarker interpretation13; and
- Retinsight’s automated algorithm performance reached area under the curve values of 0.93 and 0.85 for intraretinal fluid and 0.87 for subretinal fluid in the central millimeter.14
Why These Tools Face a Higher Bar Than DR Screening
Unlike autonomous DR systems, assistive CDS platforms do not yet carry standalone CMS screening reimbursement and typically remain embedded within bundled clinical or research workflows. Because the technology informs treatment planning over time instead of issuing a single screening decision, it faces higher regulatory and validation thresholds across multiple disease stages, devices, and imaging conditions.
BEYOND THE RETINA: ANTERIOR SEGMENT AND NEURO-OPHTHALMIC APPLICATIONS
Anterior Segment Imaging and Surgical Planning
For the assessment of angle closure and anterior segment–driven surgical planning, AI modules integrated into anterior segment OCT devices can quantify anterior chamber depth and volume, angle opening distance, trabecular meshwork–to–iris space area, iris configuration, lens position, and corneal parameters. One example is the Visulytix/CASIA2 platform (Tomey). This quantitative information can support cataract and phakic IOL surgical planning, glaucoma surgical planning, and postoperative monitoring following MIGS and other angle procedures.
Eye Movement–Based Concussion Risk Assessment
AI-enabled tracking of eye movement can be used to analyze cranial nerve–related function and generate a risk assessment score. For example, EyeBox (Oculogica) is used across sports medicine practices, neurology clinics, and eye care practices. The device presents a video stimulus while tracking eye movements and uses the collected data to estimate the likelihood of concussion.
HOME MONITORING: CONTINUOUS DATA BETWEEN VISITS
Beyond the clinic, home-based care is increasingly defined by continuous, longitudinal monitoring that can detect change between visits and support earlier intervention.
Glaucoma
For glaucoma management, home monitoring is focused on expanding IOP sampling beyond episodic office measurements. The iCare Home2 (Icare USA) rebound tonometer allows patients to measure their own IOP at home.15 Smart Lens is developing an IOP-sensing contact lens for home monitoring.16 Similarly, ImplantData and InjectSense are developing implantable biocompatible microsensors intended to measure IOP continuously or on demand.17 Clinical testing of these sensors is underway. If approved, the devices could generate large-scale home IOP datasets suitable for AI-enabled analytics.
Retina
In retina, home monitoring is aimed at detecting disease progression and treatment response between visits, particularly for patients with AMD. Scanly (Notal Vision), a home OCT system, supports remote structural monitoring and analytics of OCT biomarkers associated with disease progression and treatment response.18 Other approaches emphasize functional change. For example, ForeseeHome (Notal Vision) provides vernier acuity testing to support the early detection of metamorphopsia.19
EXPANDING HOME TESTING
Some platforms aim to provide at-home testing that can be deployed across multiple conditions and incorporated into long-term care workflows. RemoniHealth offers a suite of functional tests for AMD, glaucoma, DR, thyroid eye disease, and other indications20:
- Macular function. Macustat provides on-demand self-assessment of visual acuity, Amsler grid performance, and hyperacuity perimetry using consumer devices without dedicated hardware.
- Visual field and acuity. Peristat supports home perimetry for visual field testing, and Accustat supports visual acuity testing.
- Ocular surface and motility. Photostat uses AI-enabled tracking to assess ocular surface and motility features.
- Care integration. RemoniHealth has also built an enterprise platform for remote monitoring and care management that recently surpassed 100,000 RPM and PCM encounters.
DRY EYE: AI-ENABLED BEHAVIORAL SUPPORT
Emerging AI solutions in the dry eye space include consumer-facing tools aimed at symptom mitigation. The Blinkr app (Blinkr) offers an at-home or workplace approach to dry eye symptoms associated with computer use. It monitors the user’s blink rate and provides a notification when the rate falls below a threshold. The company reports that, over time, the app retrains blink behavior and alleviates symptoms.21
OPERATIONAL AI IS SCALING FASTER THAN CLINICAL AI
Some of the most immediate and scalable effects of AI in ophthalmology are occurring in practice operations rather than diagnostics. Tools are being used to streamline electronic health record documentation, revenue cycle management, coding, billing, scheduling, and ophthalmology-specific analytics across a range of platforms and vendors (Table 2).22-28
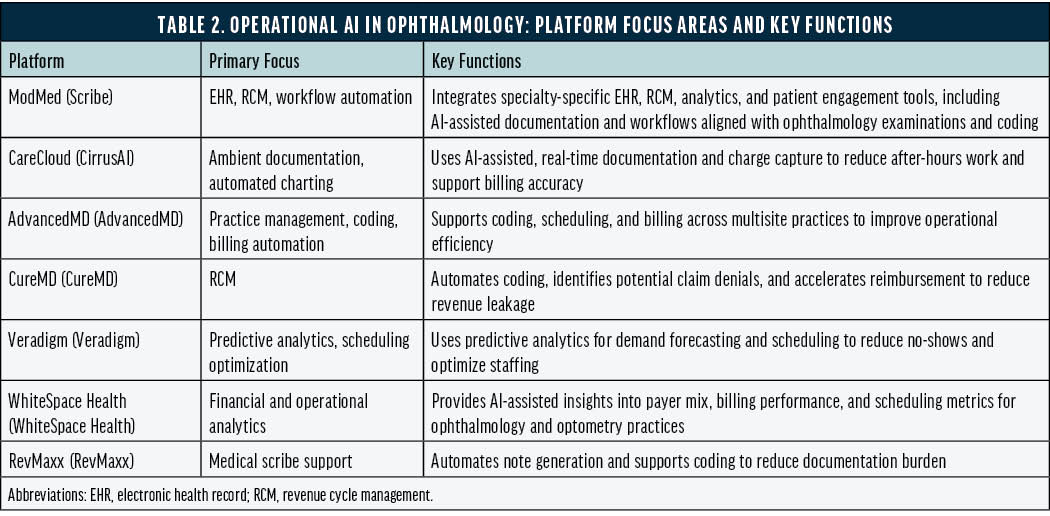
Although home monitoring is extending clinical data capture, the most widespread real-world AI deployment in ophthalmology is occurring operationally. Automation of documentation, billing, scheduling, and analytics is advancing faster than frontline clinical AI tools. Because the former typically pose a lower direct risk to patient safety, regulatory oversight is often lighter, which may accelerate adoption and enable future integration with more advanced agentic AI systems.
Agentic AI
Agentic AI represents the frontier of automation because it is designed to plan and execute multistep workflows across domains rather than to complete isolated tasks.
Patient journey workflows. Sully.ai markets AI agents spanning intake, scheduling, documentation, coding, and follow-up.29
Revenue cycle workflows. FinThrive applies agentic principles to automate claims submission, denial detection, appeals, and revenue optimization with minimal human oversight.30
Why Fully Agentic Clinical AI Has Not Arrived Yet
No fully agentic clinical platforms currently exist. Available systems remain partially autonomous, reflecting a practical constraint: as autonomy increases, regulatory scrutiny and clinical risk increase. As regulatory science evolves alongside algorithmic capability, fully agentic clinical AI may become an inevitability rather than a theoretical possibility.
1. Centers for Medicare & Medicaid Services. Calendar Year (CY) 2025 Medicare Physician Fee Schedule Final Rule. November 1, 2024. Accessed January 2, 2026. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rule
2. Centers for Medicare & Medicaid Services. Clinical Decision Support Software. Final Guidance for Industry and Food and Drug Administration Staff. September 28, 2022. Accessed January 2, 2026. https://www.fda.gov/media/109618/download
3. U.S. Food and Drug Administration. Artificial Intelligence-Enabled Device Software Functions: Lifecycle Management and Marketing Submission Recommendations. Draft Guidance for Industry and FDA Staff. January 7, 2025. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/artificial-intelligence-enabled-device-software-functions-lifecycle-management-and-marketing
4. U.S. Food and Drug Administration. Marketing Submission Recommendations for a Predetermined Change Control Plan for Artificial Intelligence-Enabled Device Software Functions. December 4, 2024. Accessed January 2, 2026. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/marketing-submission-recommendations-predetermined-chan
5. Berg Insight. mHealth and Home Monitoring: 12th ed. 2024. Accessed January 6, 2026. https://www.berginsight.com/mhealth-and-home-monitoring
6. Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS Report Number 10. Ophthalmology. 1991;98(5):786-806.
7. Poschkamp B. Real-world challenges in AI-based diabetic retinopathy screening. Invest Ophthalmol Vis Sci. 2025;66(8):3898.
8. Wang TW, Luo WT, Tu YK, Chou YB, Wu YT. Diagnostic accuracy of EyeArt for fundus-based detection of diabetic retinopathy: a systematic review and meta-analysis. Am J Ophthalmol. 2026;281:465-479.
9. Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39.
10. Ipp E, Liljenquist D, Bode B, et al. Pivotal evaluation of an artificial intelligence system for autonomous detection of diabetic retinopathy. JAMA Netw Open. 2021;4(11):e2134254.
11. U.S. Food and Drug Administration. 510(k) Substantial Equivalence Determination Decision Summary: AEYE-DS. K221183. November 9, 2022. https://www.accessdata.fda.gov/cdrh_docs/pdf22/K221183.pdf
12. EssilorLuxottica. EssilorLuxottica acquires RetinAI, accelerating transformative AI and data management in eyecare. October 17, 2025. Accessed January 2, 2026. https://www.essilorluxottica.com/cap/content/262506
13. Altris AI. Altris AI technical specifications and performance data. In: International Catalogue 2025. OptoHellas; 2025:14-15. Accessed January 2, 2026.
https://optohellas.com/wp-content/uploads/2024/08/International-Catalogue-2025-Website.pdf
14. Pawloff M, Wagner SK, Schlegl T, et al. Performance of retinal fluid monitoring in OCT imaging by an automated deep learning algorithm in clinical routine. Br J Ophthalmol. 2024;108(11):1538-1544.
15. iCare World. iCare HOME2: a modern approach to diurnal IOP monitoring. Accessed January 2, 2026. https://www.icare-world.com/us/product/icare-home2
16. Kazanskiy NL, Khonina SN, Butt MA. Smart contact lenses—a step towards non-invasive continuous ocular health monitoring: a review. Biosensors (Basel). 2023;13(10):931.
17. Wu KY, Wu KY, Wu KC, et al. Advancements in wearable and implantable intraocular pressure biosensors for glaucoma management: a comprehensive review. Micromachines (Basel). 2023;14(10):1915.
18. Notal Vision. FDA grants AI-powered Notal Vision Home OCT “SCANLY” de novo marketing authorization. Published May 15, 2024. Accessed January 2, 2026.
https://notalvision.com/assets/press-releases/May-16-2024-FDA-Grants-AI-Powered-Notal-Vision-Home-OCT-22SCANLY22-De-Novo-Marketing-Authorization.pdf
19. Chew EY, Clemons TE, Bressler SB, et al; AREDS2-HOME Study Research Group. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology. 2014;121(2):535-544.
20. RemoniHealth. RemoniHealth completes 80,000 remote monitoring encounters for chronic retina diseases. December 18, 2024. Accessed January 2, 2026.
21. Blinkr. How Blinkr works: an AI-powered blink reminder for PC. Accessed January 2, 2025. https://getblinkr.com
22. Modernizing Medicine. All-in-one ophthalmology EHR/EMR software suite. Accessed January 2, 2026. https://www.modmed.com/specialties/ophthalmology
23. CareCloud. cirrusAI Notes: AI-powered clinical documentation. Accessed January 2, 2026. https://carecloud.com/cirrusai-notes
24. AdvancedMD. Multi-location medical practice software. Accessed January 2, 2026. https://www.advancedmd.com/group-practice/multi-site-management
25. CureMD. Revenue cycle management (RCM) software. Accessed January 2, 2026. https://www.curemd.com/rcm-software.asp
26. Veradigm. Reducing no-shows and increasing ROI in Veradigm. Curogram. Accessed January 2, 2026. https://curogram.com/blog/emr-integration/veradigm/reduce-no-shows-veradigm
27. WhiteSpace Health. Ophthalmology and optometry AI analytics platform. Accessed January 2, 2026. https://whitespacehealth.com/ophthalmology-and-optometry-analytics
28. RevMaxx. AI medical scribe for clinical documentation. Accessed January 2, 2026. https://www.revmaxx.co/
29. Sully.ai. About us. Accessed January 2, 2026. https://www.sully.ai/about-us
30. FinThrive. FinThrive introduces agentic AI at HFMA 2025 to help customers transform healthcare revenue cycle management performance. June 21, 2025. Accessed January 2, 2026. https://finthrive.com/news/finthrive-introduces-agentic-ai-at-hfma-2025-to-help-customers-transform-healthcare-revenue-cycle-management-performance




