Monofocal IOLs
P. Dee Stephenson, MD, FACS

It is sometimes surprisingly difficult to pick the most appropriate lens for a patient. Many options are available—some with negative aberrations, some with positive aberrations, and some that are aberration-free (aspheric). The postoperative quality of vision attained makes IOL selection worth the hard work it requires.
The average spherical aberration of the anterior corneal surface is +0.27 µm. I achieve the best results for patients when the cornea has positive aberrations postoperatively. I believe that IOL material matters. Some IOLs have glistenings, some have ridges on the optic edge, and some rotate more than others. I have used almost every IOL platform, mixed and matched many of them, and have found the most success with monofocal IOLs—specifically, the enVista aspheric IOL and the enVista aspheric monofocal toric IOL (Bausch + Lomb).
A Strong Preference
The enVista lenses have the same power from their center to their edge, and they induce no spherical aberrations. In my hands, these IOLs are forgiving, with little in the way of dysphotopsias and increased depth of field postoperatively. In my practice, 86.4% of patients who have received these IOLs have achieved postoperative uncorrected intermediate visual acuity of 20/40 or better, and they have been able to use handheld devices and perform some near-vision tasks without glasses (Figure 1A). They have some uncorrected near visual acuity as well (Figure 1B). Because patients retain consistent uncorrected distance visual acuity across a wide range of manifest refraction spherical equivalent, they tolerate defocus well (Figure 2). In my experience, the enVista aspheric monofocal toric IOL is also a great choice for patients with aberrated corneas because this lens does not induce spherical aberration and offers a large range of cylinder correction (+0.77 to +4.53 D).
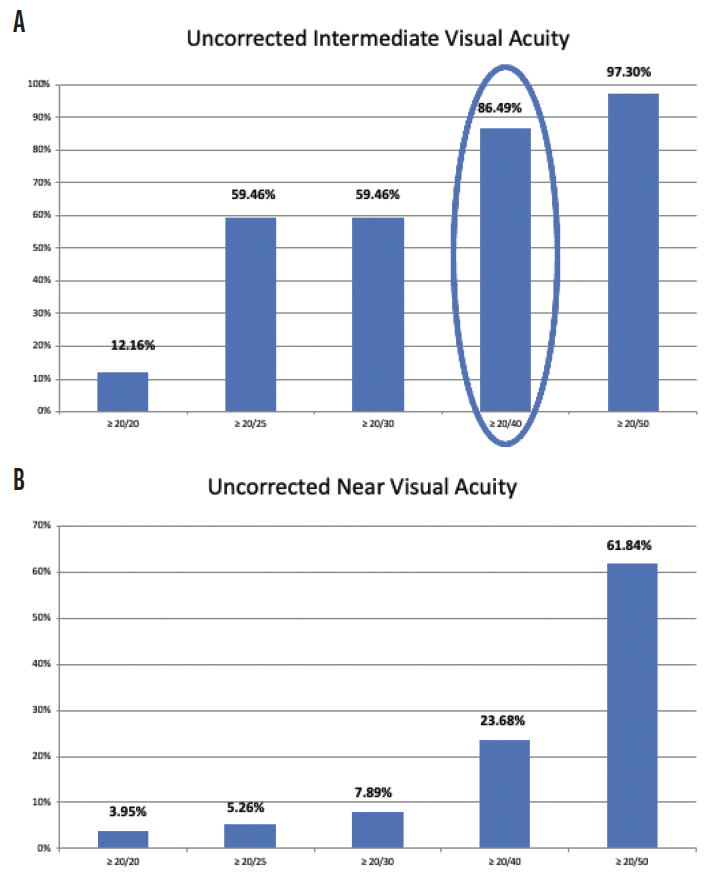
Figure 1. Postoperative uncorrected intermediate visual acuity (A) and postoperative uncorrected near visual acuity (B) among patients who received an enVista monofocal toric IOL in Dr. Stephenson’s practice.
Figures 1–3 courtesy of P. Dee Stephenson, MD, FACS
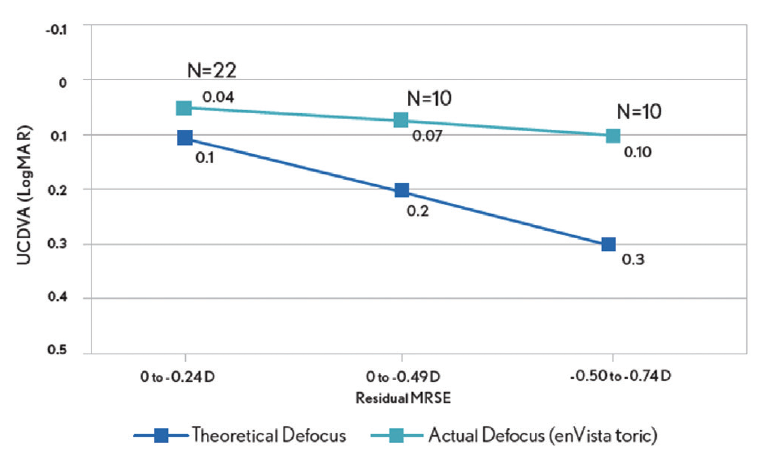
Figure 2. Postoperative tolerance of defocus among patients who received an enVista monofocal toric IOL in Dr. Stephenson’s practice.
When I compared my results with low-add toric IOLs and monofocal lenses, I found that using the former improved clinical outcomes and patient satisfaction and expanded the treatment options for cataract patients with a low amount of corneal astigmatism (Figure 3).
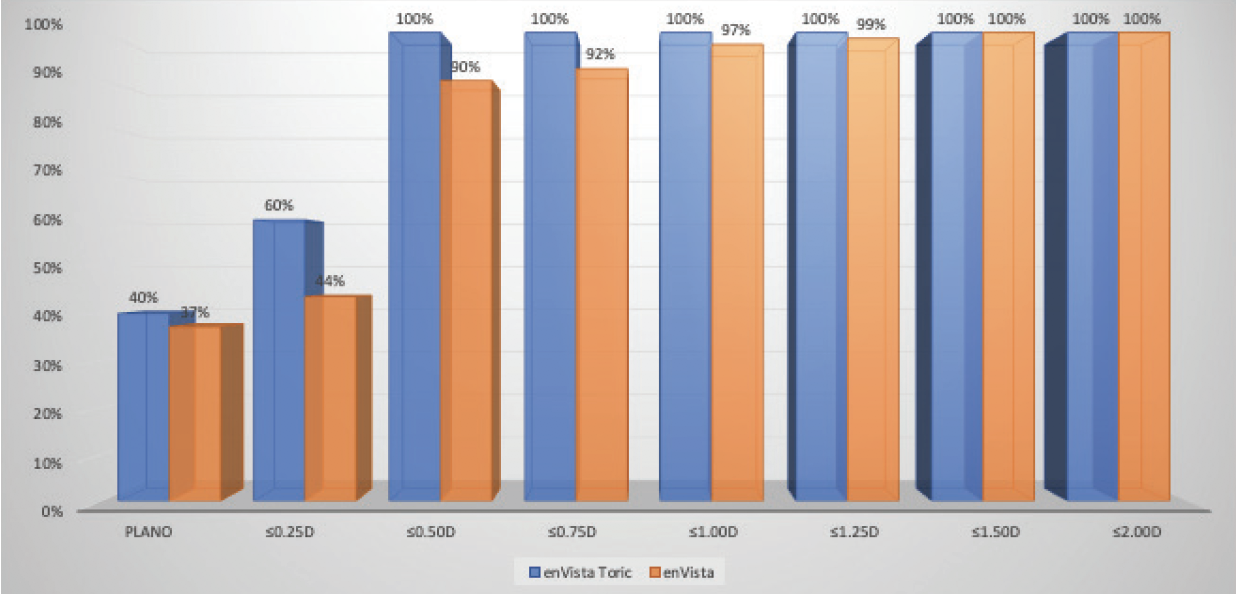
Figure 3. Dr. Stephenson’s comparison of postoperative refractive astigmatism in cataract patients with a low amount of corneal astigmatism after the implantation of an enVista toric IOL versus an enVista monofocal IOL.
Conclusion
I work hard to optimize my surgical outcomes, honing my surgical skills and preoperative workups and doing everything from correcting dry eye disease and identifying preexisting ocular surface to addressing retinal disease. Monofocal IOLs, specifically the enVista aspheric and monofocal toric IOLs, allow me to enhance my patients’ lives with stable, predictable outcomes and great quality of vision.
Multifocal IOLs
Kevin L. Waltz, OD, MD

Cataract surgery with a high-quality monofocal IOL is a marvel that can achieve great outcomes when executed well. The cost of the procedure is minimal to most patients who undergo it, and they usually can expect to see well at distance without spectacles. In my experience, some of these patients will be able to read without correction as well and will be able to function without glasses as much as 60% of the time.
Premium IOLs are competing against a remarkably safe, cost-effective, and efficient surgery, and these lenses are relatively expensive for most patients. If they pay the required fee, they expect the result promised. That’s why I prefer multifocal IOLs.
If a standard IOL provides great distance vision and some functional near vision in each eye, a multifocal IOL must deliver much more. The surgeon and the process must deliver good vision at more than one distance. Multifocal IOLs provide good distance and near vision—perhaps with some level of intermediate vision—in each eye. A monovision approach cannot provide that level of vision. It compromises at least one eye’s distance vision; therefore, to satisfy a patient, the surgeon must significantly modify the patient’s expectations.
The choice boils down to the following: good distance vision possibly with some halos and glare but the ability to read or blurry distance in one eye and blurry near vision in the other. Of the currently available technologies, multifocal IOLs are a great option. Patients want to be confident, however, that they will get what they pay for, and it is the surgeon’s job to ensure it (see Embrace Enhancements).
EMBRACE ENHANCEMENTS
One way to improve patient and surgeon satisfaction is to fine-tune the original surgery’s outcome. In the 1990s, LASIK had about a 30% enhancement rate for several years for various reasons, but virtually every patient was happy with their results. A few years later, the enhancement rate decreased to 10%, and patients were generally less happy with their outcomes. They tolerated the extra surgery but were, in general, more resistant to undergoing an enhancement. At issue were expectations. When the rate was 30%, enhancements were expected and therefore viewed by patients as normal. At 10%, enhancements were not expected and therefore viewed as abnormal.
A similar evolution has occurred with premium IOLs. From 1998 until 2005, I implanted many presbyopia-correcting IOLs in patients without charging them extra for the services. Their expectations were similar to those for standard cataract surgery, in part because they did not pay extra for the service. Preoperative discussions were less intensive, outcomes were not as good, and patient satisfaction was higher than they currently are in my practice. My patients pay a significant premium for presbyopia correction whether or not cataract surgery involves the use of a femtosecond laser. Compared to patients from the earlier era, modern patient expectations tend to be higher and their level of satisfaction lower.
Comfortably offering any form of presbyopia correction to patients requires bundling its costs into the package of services. The surgeon must be able to offer an enhancement strategy. This is true whether the original offering is monovision or a presbyopia-correcting IOL. Claims that a refractive enhancement is never needed should be abandoned. Outcomes will not be perfect on the first try every time. A good surgeon will likely have an enhancement rate of approximately 10% regardless of the IOL strategy, and the surgeon, staff, and patient should plan on that.
EDOF IOLS
James C. Loden, MD

When first asked to discuss why I prefer extended depth of focus (EDOF) lenses over monovision or multifocal lenses, I responded that I could not take that position in good faith because surgeons at a full-service refractive practice must tailor their recommendations to the individual needs of their patients, not the surgeons’ personal biases. Each of these lens designs will be appropriate and perform well in certain situations. Herein are the scenarios in which I prefer to use an EDOF IOL over mono- and multifocal options.
Strengths and Weaknesses
In my opinion, loss of light is a major source of patient dissatisfaction with multifocal lenses that have truly diffractive optics. They lose approximately 18% to 20% of light. This reduction is more evident in my refractive lensectomy patients than in my cataract patients, especially among those who require fine color and shading discrimination. In general, between 8% and 10% of available light is lost with EDOF lenses. In my experience, patients do not perceive this reduction.
Dysphotopsias are arguably the greatest obstacle to the higher adoption of presbyopia-correcting lenses. Debilitating glare, starbursts, and halos at night have necessitated the explantation of multifocal lenses in about 2% of my patients. Patients of mine who have received EDOF lenses are far less likely to report dysphotopsia. These lenses provide high-quality distance and intermediate vision but inadequate near vision for spectacle independence. Rarely, I have explanted both EDOF models for intractable dysphotopsia.
The Case for EDOF IOLs
Patient selection. During IOL selection, the patient’s history is of the utmost importance. Their profession and social activities are major factors. Do they like to hike, hunt, fish, or play golf or tennis? Do they operate heavy equipment or drive for hours at night? Are they comfortable wearing +1.00 to +1.50 D readers to view fine print, spreadsheets, and paperback books? Have they ever worn monovision or multifocal contact lenses? If so, how did they tolerate the experience?
EDOF lenses serve my outdoorsy patients well. Activities such as hunting, fishing, and shooting prioritize distance and intermediate vision over near vision. Outdoor photographers require near vision that is adequate to see their camera display without a loss of contrast sensitivity. High-quality night vision and low-light vision are a priority.
I find that EDOF lenses also work well for truckers, farmers, private pilots, and operators of heavy equipment because they need high-quality, dysphotopsia-free distance vision and crisp intermediate vision. They generally find occasionally using low-powered readers to be acceptable.
Presence of mild ocular pathology or amblyopia. EDOF lenses can be an excellent option for patients with mild ocular pathology or amblyopia. I recently implanted an EDOF lens in the amblyopic eye of a patient who underwent refractive lens exchange for hyperopic presbyopia and a multifocal lens in the nonamblyopic eye. The patient was very satisfied with blended vision.
Multiple studies have reported good outcomes with EDOF lenses in patients with mild ocular pathology such as anterior basement membrane dystrophy, epiretinal membrane, drusen, and amblyopia. A thorough preoperative discussion that sets realistic expectations is mandatory.
Primary mini-monovision enhancement. EDOF IOLs may be used for a primary mini-monovision enhancement in patients who are dissatisfied with their near vision. Targeting a postoperative refraction of -0.75 to -1.00 D in the nondominant eye can achieve exquisite intermediate and near vision while avoiding having to set a eye to a -2.00 D intended refraction. On several occasions, I have performed a +1.00 D LASIK enhancement on a plano eye to improve uncorrected near visual acuity to J1+.
EDOF IOL Downsides
There are two drawbacks to EDOF lenses. The first is that they provide inadequate near vision to read fine print. The second is dysphotopsia inherent to all diffractive optics. A PRK or LASIK enhancement can often address both of these complaints, as in the example of mini-monovision discussed earlier. Before proceeding with an enhancement, I conduct a contact lens trial or free lenses in a trial frame to determine if an enhancement will resolve the issue and how much refractive correction is required. The greatest source of dissatisfaction with refractive surgery is ametropia. Even 0.50 D of residual cylinder can disappoint patients and compromise their neural adaptive ability.
Targeting a postoperative refraction of -0.75 to -1.00 D in the nondominant eye achieves exquisite intermediate and near vision. Patients often tolerate the -0.75 to -1.00 D difference between the mini-monovision eye and the dominant distance and intermediate eye much more easily than they might tolerate the 2.00 D difference between eyes that is often necessary to give J1+ uncorrected near vision using a traditional monofocal lens for monovision.
1. Tecnis Symfony IOL. Johnson & Johnson Surgical Vision. Accessed December 14, 2020. /www.jnjvisionpro.com/products/tecnis-symfony%C2%AE-iol
A CLOSER LOOK AT OTHER OPTIONS
Initial Experience With the Light Adjustable Lens
By Marilyn Zuniga, OD, and William B. Trattler, MD


Patients have high expectations when undergoing cataract surgery. Advances in IOL formulas and in IOL technology have improved visual outcomes over the past 5 years. Refractive surprises can occur nevertheless—particularly if corneal curvature or axial length is atypical or the patient has a history of corneal surgery. An option for these patients is the Light Adjustable Lens (RxSight). This technology provides the unique opportunity to modify the refractive error during the postoperative period to meet their functional needs.
At our center, we have found the Light Adjustable Lens to be helpful for a significant number of patients, including those with a history of corneal surgery such as LASIK, PRK, SMILE, and corneal transplantation. This IOL can also be an excellent option for patients with irregular astigmatism due to keratoconus and those with mild corneal opacities for whom it is hard to determine the true corneal power. Patients with long and short eyes, steep or flat corneas, up to 3.00 D of astigmatism, and other anatomical variations can also benefit. Those who desire monovision or blended vision are also excellent candidates.
Our initial experience with the Light Adjustable Lens has been positive. Most of our patients who have chosen this technology have a history of corneal refractive surgery or mild irregular astigmatism. UCVA is typically 20/25 or better among those who have the goal of excellent distance vision. Our patients who prioritize intermediate or near vision have achieved their target refractions after adjustments. In one of the more challenging cases, the patient had irregular astigmatism from a small corneal opacity (Figure 1). After the adjustments, UCVA was 20/25, and the patient was thrilled with the outcome (Figure 2).
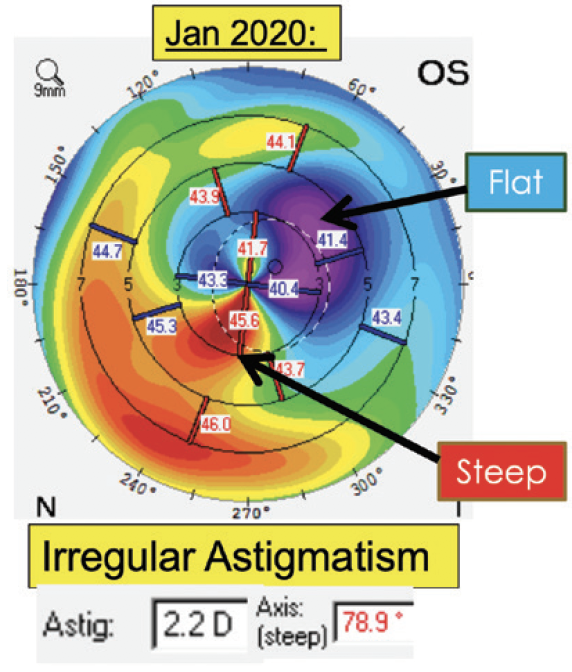
Figure 1. A patient with a history of a small corneal opacity that resulted in irregular astigmatism who received an Light Adjustable Lens.
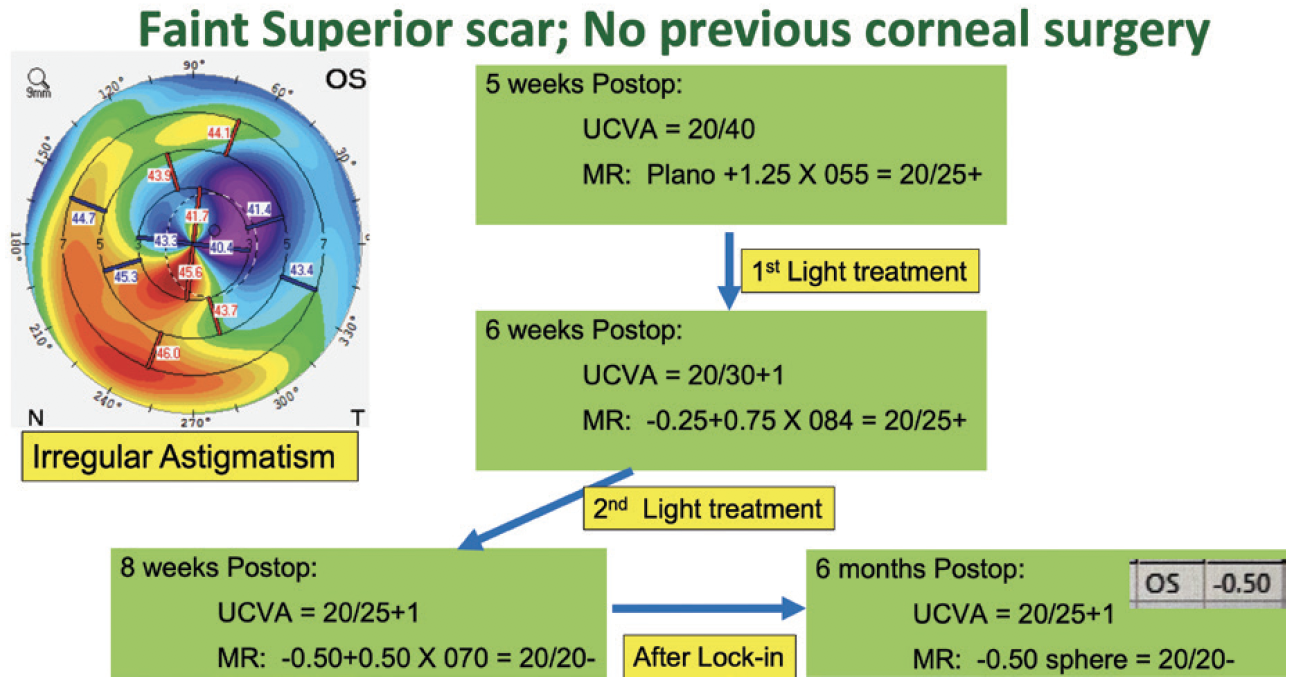
Figure 2. Following adjustments, UCVA was 20/25.
A CLOSER LOOK AT OTHER OPTIONS (continued)
Chromophore IOLs: What Does the Evidence Say?
By Daniel H. Chang, MD

Chromophore IOLs that filter high-energy light can be both beneficial and detrimental to ocular and systemic health. With the widespread evolution of artificial lighting to LED sources, our understanding and implementation of chromophore IOLs must also advance. High-energy short-wavelength light describes a wide swath of the visible light spectrum, including violet (380–460 nm) and blue (460–500 nm) light. Traditional descriptions of chromophore IOLs as yellow versus clear and the oversimplified distinctions of blue-light– versus violet-light–filtering are too vague and only minimally helpful.
To understand the full impact of high-energy light-filtering chromophores, we must consider both the shape of the transmission spectrum and the role that specific wavelengths play in ocular and systemic health. In a recently published article, my colleagues and I reviewed the literature on violet- and blue-light–filtering chromophores.1
EVALUATING CHROMOPHORE IOLS
The three primary areas of consideration when evaluating chromophore IOLs may be summarized as follows.
Visual quality and function. Within the visible light spectrum, high-energy short-wavelength light causes greater scatter (contributing to dysphotopsias), induces more chromatic aberration,2 and causes more visual discomfort. Chromophore IOLs can improve night vision symptoms by reducing light scatter.3 However, because blue light plays an important role in scotopic vision,4 chromophores that filter blue light can have a negative effect on vision in low light. Color perception can also be affected.
Phototoxicity. Short-wavelength, high-energy light also contributes to oxidative stress in the macula by producing reactive oxygen species. Most studies of macular phototoxicity simply group violet and blue light together. In a study that evaluated narrow bands of light, however, the greatest amount of reactive oxygen species formation associated with retinal pigment epithelial cell damage was caused by violet light in the range of 415 to 455 nm, with a peak at 420 nm.5 For optimal photoprotection, violet light should therefore be maximally filtered. Blue-light filtering can also be protective but to a lesser degree.
Circadian rhythm. The circadian rhythm is important for cognition, sleep, metabolism, and many other aspects of physiological health. It is entrained by nonvision-related cells in the retina, known as intrinsically photosensitive retinal ganglion cells. When these cells are stimulated by blue light, particularly in the range of 460 to 480 nm, they release melanopsin, which suppresses melatonin, the hormone driving circadian rhythm function.4,6 Studies have shown that the implantation of a clear IOL improves sleep quality and cognition, but reports have been inconsistent regarding the impact of blue-light–filtering chromophore IOLs on the circadian rhythm.
TRADEOFFS
As with many aspects of IOL design, the ideal transmission profile of chromophores involves a balance of tradeoffs in visual quality and function, phototoxicity, and circadian rhythm. No chromophore is ideal under all circumstances. I would argue that the best approach is to block wavelengths that are known to be detrimental and to transmit wavelengths that may be beneficial. Because of an overlap in the benefits and drawbacks of high-energy wavelengths, it is helpful for a chromophore to have a steep cutoff in the transmission spectrum (Figure).
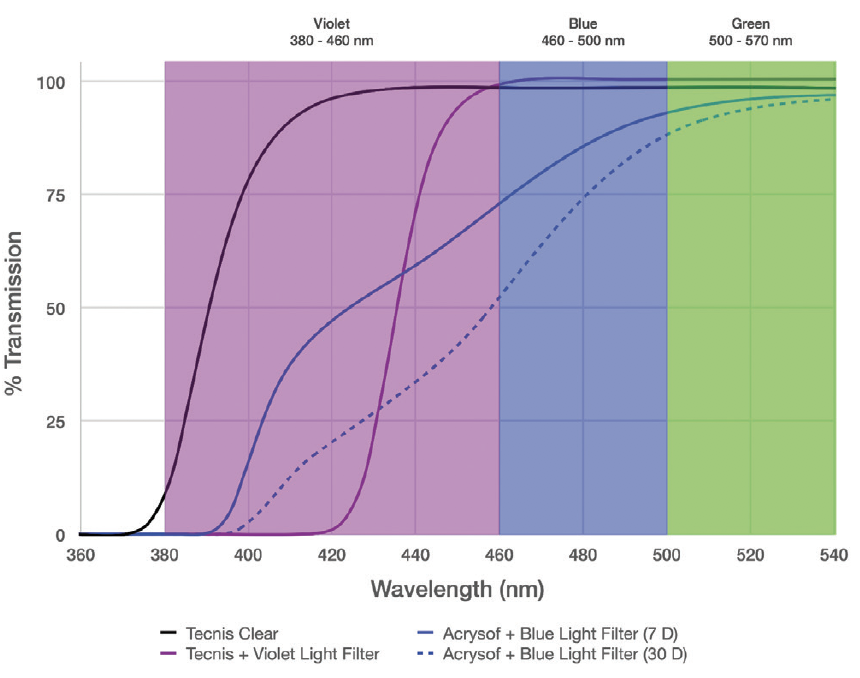
Figure. Transmission spectra of various IOLs, including those with violet-light– and blue-light–filtering chromophores. Note the steep IOL power–independent cutoff of the violet-light filter versus the broad cutoff of the blue-light filter, which moderately reduces the transmission of important blue light while allowing the transmission of high-energy phototoxic violet light, especially in lower-diopter IOLs.
Both violet and blue light cause light scatter and phototoxicity, but blue light is also important for scotopic vision and circadian rhythm entrainment. It would therefore be optimal to filter violet wavelengths below 420 nm and to transmit blue wavelengths above 450 nm. For extra protection against phototoxicity from blue light, sunglasses can be worn during the daytime and removed when indoors and at nighttime, thus limiting a negative impact on scotopic vision, circadian rhythm function, and color perception.
1. Chang D, Pastuck T, Rosen R, et al. Violet and Blue Light: Impact of High-Energy Light on Vision and Health. J Ophthalmic Stud. 3(2): dx.doi.org/10.16966/2639-152X.119.
2. Zhao H, Mainster MA. The effect of chromatic dispersion on pseudophakic optical performance. Br J Ophthalmol. 2007;91:1225-1229.
3. Hammond BR Jr, Renzi LM, Sachak S, Brint SF. Contralateral comparison of blue-filtering and non-blue-filtering intraocular lenses: glare disability, heterochromatic contrast, and photostress recovery. Clin Ophthalmol. 2010;4:1465-1473.
4. Mainster MA. Violet and blue light blocking intraocular lenses: photoprotection versus photoreception. Br J Ophthalmol. 2006;90(6):784-792.
5. Marie M, Bigot K, Angebault C, et al. Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell Death Dis. 2018;9(3):287.
6. Brøndsted A, Lundeman J, Kessel L. Short wavelength light filtering by the natural human lens and IOLs—implications for entrainment of circadian rhythm. Acta Ophthalmol. 2013;91:52-57.








