CASE PRESENTATION
An 80-year-old man presents with blurred vision in his left eye that has worsened progressively over the past 4 weeks. He reports no pain or physical discomfort but says the blurriness is causing nausea.
The patient underwent bilateral cataract surgery 5 years ago and has no history of trauma. He is not currently administering IOP-lowering topical drops but says he did so in the past.
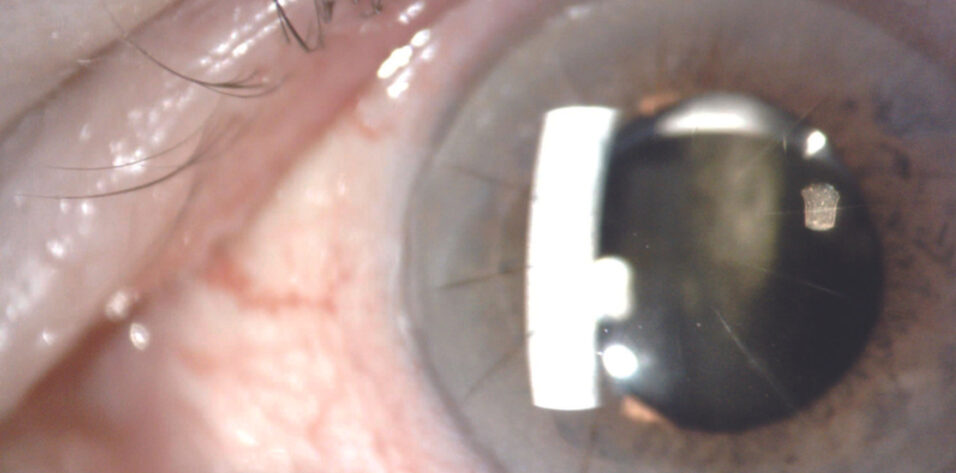
On examination, the patient’s visual acuity is 20/25 OD and 20/200 OS. His BCVA with manifest refraction is 20/20 OD and 20/150 OS. IOP readings with applanation tonometry are 19 mm Hg OD and 27 mm Hg OS. A slit-lamp examination finds poor pupillary dilation (about 4.5 mm), transillumination defects, and mild vitreous prolapse forward in both eyes. The one-piece IOL in the left eye is inferiorly displaced in the bag. A dilated fundus examination and macular OCT imaging show a mild epiretinal membrane in each eye and cup-to-disc ratios of 0.3 OD and 0.4 OS.
Therapy with a fixed combination of brinzolamide 1% and brimonidine tartrate 0.2% ophthalmic solution (Simbrinza, Alcon) is initiated in the left eye. The patient is asked to obtain his old medical records for a follow-up visit later the same week. At that visit, the IOP is 12 mm Hg OS. The records document a history of pseudoexfoliation (PXF) syndrome and high IOP in both eyes following cataract surgery. The operative report describes uneventful cataract surgery, the use of a pupillary expansion device, and the implantation of a one-piece hydrophilic acrylic IOL (Softec One IOL, Lenstec) in each eye. OCT imaging shows normal optic nerves. Measurements with the OPD-Scan III (Nidek) find more than 8.00 D of lens-based cylinder in the left eye (Figure 1). Pachymetry reveals asymmetric corneal thickness between the two eyes (Figure 2).
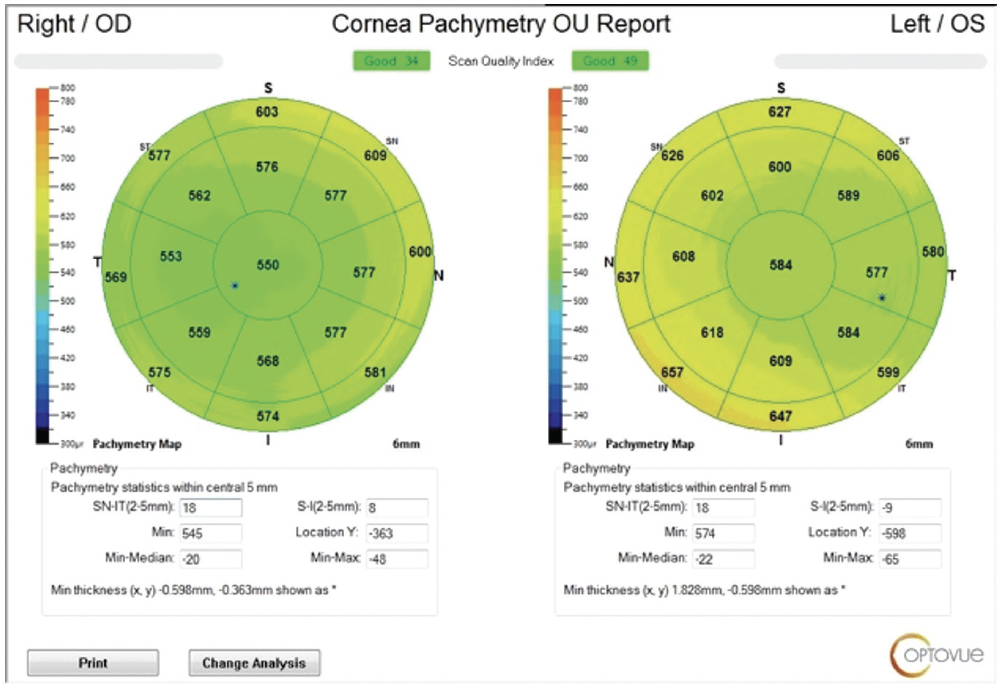
Figure 1. The OPD-Scan III measures 8.00 D of lens-based cylinder in the affected eye.
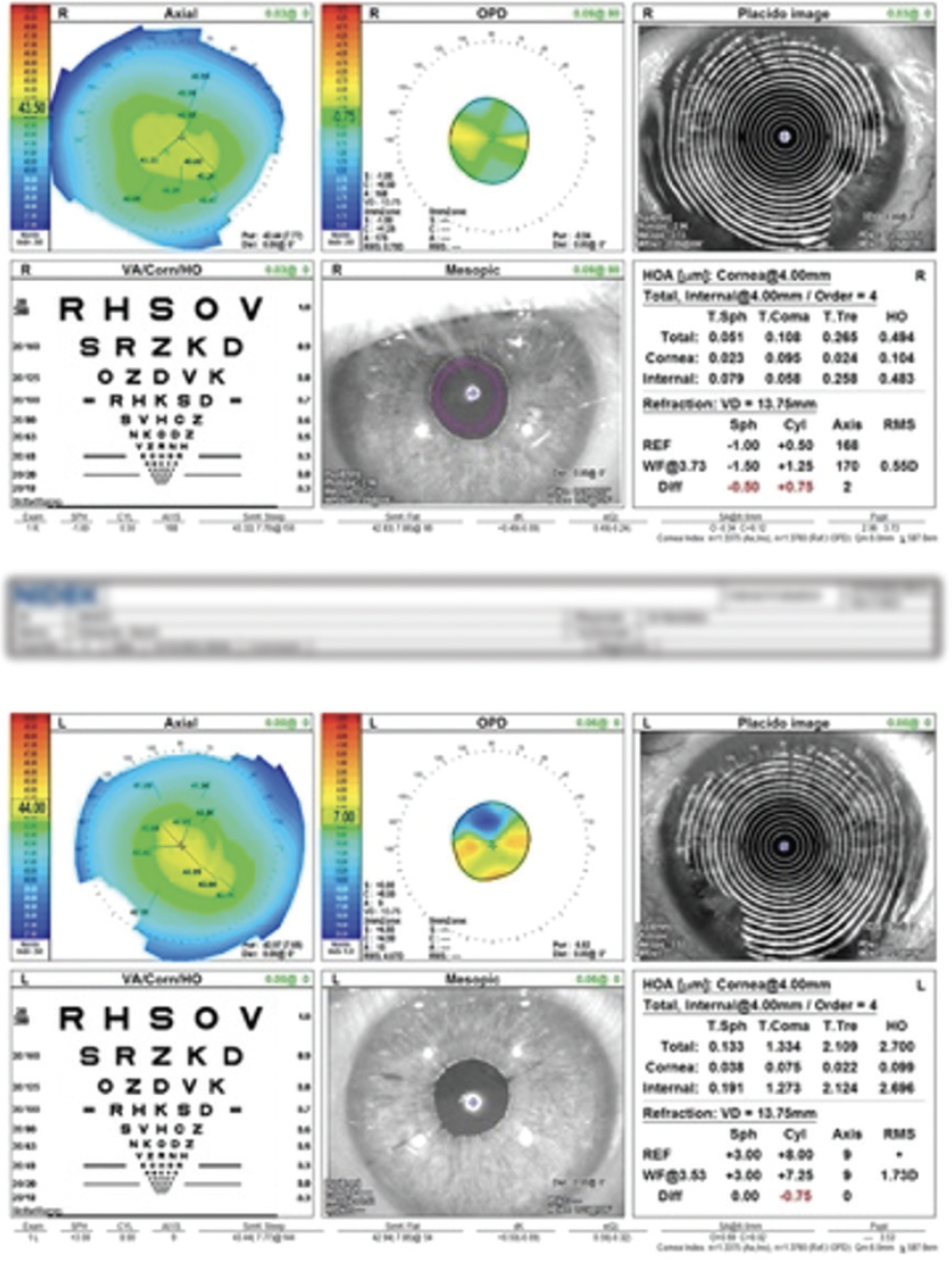
Figure 2. OCT imaging shows asymmetry in central corneal thickness between the patient’s two eyes. The cornea of the affected eye is thicker than average.
How would you approach surgery? Would you recommend additional testing or examination before surgery? How would you counsel the patient?
— Case prepared by Cristos Ifantides, MD, MBA

SARA BOZORG, MD
At Maine Eye Center in Portland, Maine, where I practice, we take a team approach to the management of dislocated IOLs. One day every week is designated for these appointments. Patients are informed in advance that they will consult with both the retina team (my colleague Jeffrey Moore, MD) and the anterior segment team (me). In this way, we can bring our shared expertise to surgical planning.
The patient in this case would undergo a thorough examination of the peripheral retina, macular OCT imaging to assess posterior vitreous detachment status, an evaluation of the IOL’s position while he is supine, and biometry. I would also consider the white-to-white distance when deciding between intrascleral haptic fixation (ISHF) with the Yamane technique1 and a scleral-sutured one-piece IOL (CZ70BD, Alcon). I tend to favor the latter approach for eyes that have a white-to-white distance greater than 12.6 mm.
Before proceeding to surgery, I would have a frank discussion with the patient about the BCVA in his left eye and the possibility of additional corneal edema with more surgery. I would also discuss the need for astigmatism correction with glasses or a contact lens after surgery.
I would offer the patient the following three options and explain their risks and benefits:
- Option No. 1: Observation. Surgery would not be performed. IOP control would be discussed. The patient would be monitored closely until the IOL (likely) drops, at which time he could begin wearing an aphakic contact lens. In situations like this, I usually let the patient know that this strategy is not my top recommendation, especially when vitreous is present in the anterior chamber, but that it is an option with careful observation if they are absolutely opposed to undergoing surgery.
- Option No. 2: Repositioning of the IOL. The procedure would be performed with a lasso suture and combined with a one-port anterior pars plana vitrectomy (PPV). Generally, I recommend this strategy only if the IOL is mildly displaced within the bag and a large Soemmering ring is absent, because it may rub against the iris and tilt the lens.
- Option No. 3: Combined surgery. A full PPV, an IOL exchange, and ISHF of the IOL would be performed (Figures 3 and 4).
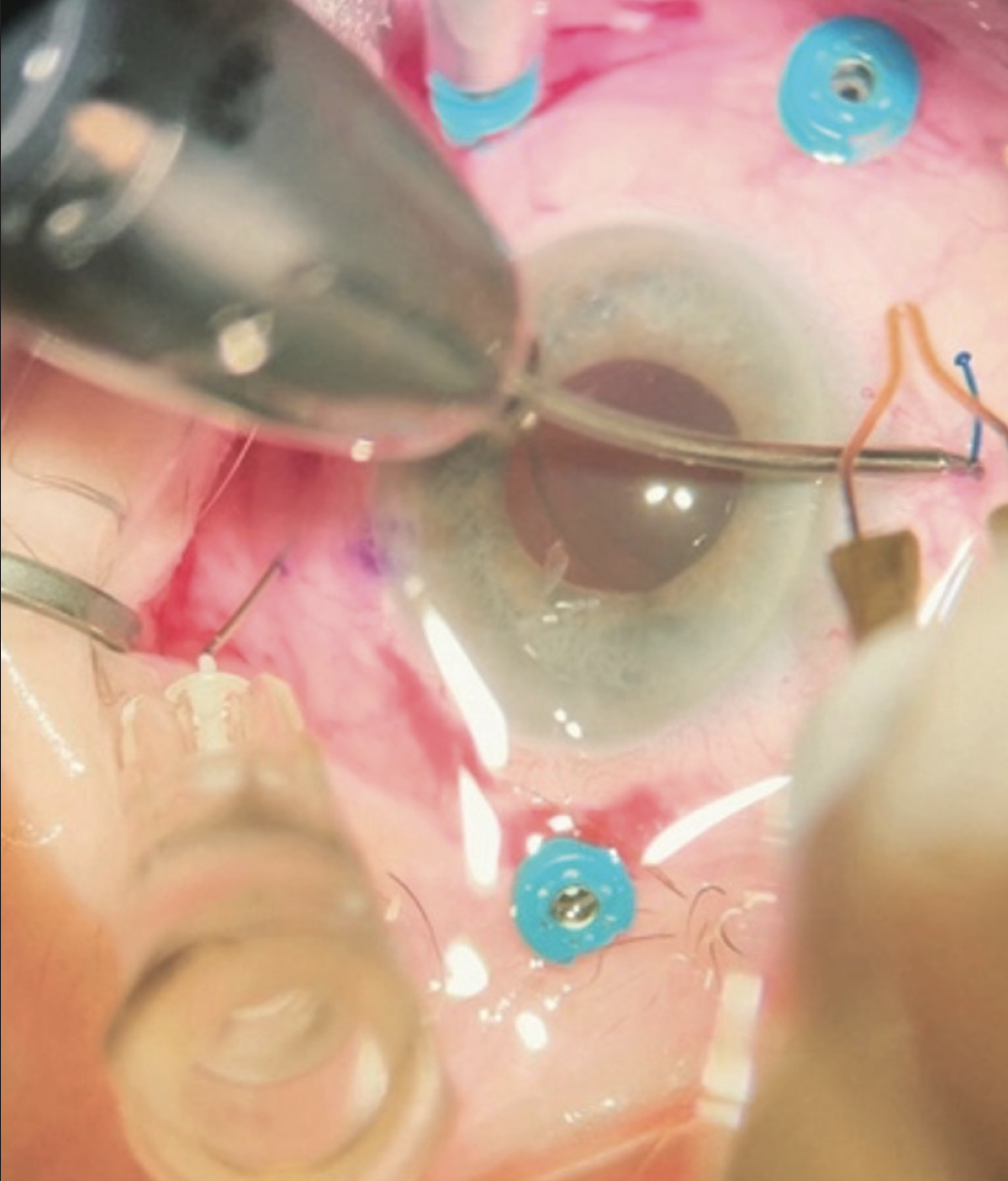
Figure 3. The setup for combined surgery where Dr. Bozorg practices. The pars plana ports are positioned out of the way of the ISHF passes in a patient’s left eye.
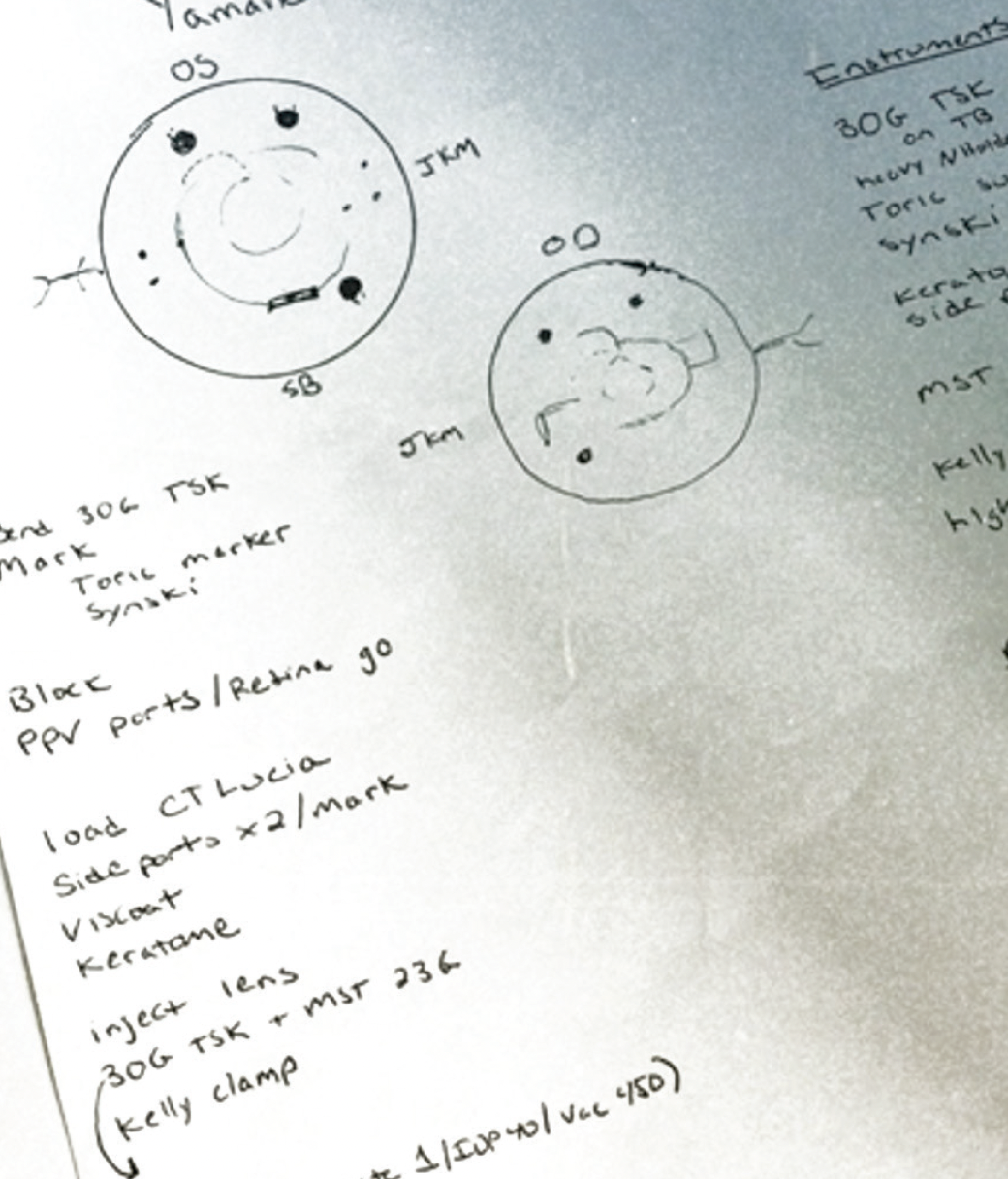
Figure 4. Dr. Bozorg and her colleagues take a team approach to managing dislocated IOLs. A retina surgeon sits superiorly, and an anterior segment surgeon sits temporally.
(Figures 3 and 4 courtesy of Sara Bozorg, MD)
I am inclined to recommend the third option because of the prolapsed vitreous, the patient’s history of PXF, the possible presence of mild corneal edema, and the likelihood that the IOL will fall back to the retina by the time of surgery. My colleagues and I do not currently incorporate microinvasive glaucoma surgery into our combined procedures, but this could be considered to address the patient’s ocular hypertension.

BRENTON FINKLEA, MD
The patient’s multiple comorbidities necessitate a thoughtful approach. A dilated examination would be performed while he is reclined in the office to determine the lens’ position and whether a posterior approach is required for its retrieval. Based on the case presentation, prior IOP monitoring in the clinic may not be reliable, and glaucoma surgery may be beneficial.
Therapy with topical aqueous suppressants would be initiated. Once IOP control is established, repeat pachymetry would be performed, and an endothelial cell count would be obtained. If the count is low or persistent corneal edema is observed, simultaneous or staged Descemet stripping endothelial keratoplasty (DSEK) would be considered. Biometry measurements and IOL power calculations from the patient’s previous surgery would be reviewed because corneal thickening could skew current keratometry readings.
My inclination is to offer an IOL exchange with ISHF and goniotomy combined with either a pars plana anterior vitrectomy performed by myself or a posterior vitrectomy performed by a retina colleague depending on the lens’ position. The refractive target would be -1.00 D because the patient will likely require DSEK in the weeks to come. (This refractive outcome would not be so myopic as to cause bothersome anisometropia if he defers further surgery.) DSEK would be performed if corneal decompensation occurs following IOL exchange.
Following the IOL exchange, goniotomy, and vitrectomy, therapy with topical steroids and NSAIDs would be continued for at least 1 month. The patient would be monitored for macular edema and peripheral retinal pathology.
Patient counseling would set realistic expectations about the potential for multiple future surgeries and the necessity of long-term monitoring and management of the patient’s ocular hypertension.

BEERAN MEGHPARA, MD
A key decision point is whether the IOL should be exchanged or repositioned and refixated to the sclera. I favor the latter approach when a toric or presbyopia-correcting IOL has dislocated and I am trying to preserve a refractive outcome with which the patient was previously happy. Circumstances in which refixation is less desirable include significant (especially posterior) dislocation of the IOL and the presence of a Soemmering ring and/or vitreous prolapse.
In the current case, given the presence of vitreous in the anterior chamber, I would recommend an IOL exchange for a scleral-fixated lens combined with an anterior vitrectomy via a pars plana approach. Regardless of the surgical plan, the vitreous must be addressed before manipulation of the IOL to limit traction on the vitreous and reduce the risk of retinal tears. I prefer to access the anterior vitreous with trocars placed in the pars plana. This enables me to pull prolapsed vitreous posteriorly out of the anterior chamber. Posterior access also allows me to perform a vitrectomy behind the IOL to reduce traction before the IOL is lifted into the anterior chamber from behind. In the absence of capsular support, ISHF of the IOL with the Yamane technique would be performed. Suture fixation of the IOL would be another option.
The patient would be counseled on his need for IOP monitoring. In the setting of PXF, postoperative IOP spikes are not uncommon. A preoperative endothelial cell count would be obtained to help stratify the patient’s risk of corneal decompensation.

TANYA TRINH, MBBS, FRANZCO
Based on the case presentation, the duration of the original cataract surgery was likely lengthy. The patient’s rapid loss of vision and high amount of lens cylinder suggest subluxation of the IOL–capsular bag complex.
If the glaucoma is medically controlled, the IOL–capsular bag complex can be removed and replaced with either a sutured IOL or a lens secured via ISHF with the Yamane technique. If a capsular tension ring were present in the bag, the IOL–capsular bag complex could be repositioned with PTFE sutures (Gore-Tex, W.L. Gore & Associates).
The cornea would be marked at 180º and 2 mm radially to this point. Next, the IOL-capsular bag complex would be held in place as a triamcinolone-assisted PPV with posterior infusion is performed. The complex would then be prolapsed into the anterior chamber, bi- or trisected with microinstruments, and removed via a scleral tunnel approach. A Sensar IOL (model AR40e, Johnson & Johnson Vision) would be implanted in the anterior chamber through the scleral tunnel. ISHF with the Yamane technique would be completed after the tunnel is closed. A slightly myopic postoperative refraction would be targeted to account for the patient’s potential need for future corneal surgery.
I would monitor the iris transillumination defects during surgery and repair them if they are significant or become symptomatic in the future, or this could be safely deferred and performed in the future if the patient becomes symptomatic.
Intraoperatively, the surgical view may be compromised by preexisting corneal decompensation. Preoperatively, the patient would be counseled that he may require ultrathin Descemet stripping automated endothelial keratoplasty in the future. I would try to delay the need for corneal transplantation by initiating therapy with a topical Rho kinase inhibitor soon after the IOL exchange; this can be a useful salvage technique. Topical glaucoma therapy would be continued, and the epiretinal membranes would be monitored.

WHAT I DID: CRISTOS IFANTIDES, MD, MBA
During an examination of the patient while he was supine, the IOL in the left eye remained planar. He was therefore referred to a retina specialist, who cleared the patient for IOL removal, pars plana anterior vitrectomy, and a sutured IOL. An anterior chamber maintainer and a pars plana trocar were used to access the vitreous behind the lens and prevent it from falling posteriorly during vitrectomy.
I chose to suture an IOL because of manufacturing issues with the CT Lucia (Carl Zeiss Meditec) at the time that have since been resolved (scan the QR code to watch surgery). Implantation of an enVista MX60E IOL (Bausch + Lomb) was my preference because its eyelets can be sutured and the power of its optic is the same from its center to its edge, making potential lens decentration less of an issue. I like to suture this lens radially at 2 and 4 mm from the limbus because this works nicely with the eyelet design, can minimize IOL rotation, and improves alignment accuracy with toric models. I do not use a cow-hitch suture technique because it has been reported to increase the incidence of eyelet fractures.2 The enVista MX60E IOL has fewer points of contact than the Akreos lens (model AO60, Bausch + Lomb). Unlike the Akreos, however, the enVista lens is made of hydrophobic acrylic and is therefore a better choice for an eye that, in the future, may undergo partial-thickness corneal transplantation with an air bubble technique or the injection of a gas bubble during retinal detachment surgery.
The patient had a partially decompensated cornea, as evidenced by increased central corneal thickness. A postoperative refraction of -1.00 D was therefore targeted in case he requires a partial-thickness corneal transplant in the future.
One day after surgery, the patient’s UCVA was 20/70+ and J2 OS. The IOP was 16 mm Hg OS on a fixed combination of brinzolamide 1% and brimonidine tartrate 0.2% ophthalmic solution (Simbrinza, Alcon) administered twice daily. Timolol was added to his drug regimen to ensure adequate IOP control during the first postoperative month.
One month after surgery, the patient’s UCVA was 20/100 and J1+ OS. The IOP was 12 mm Hg on timolol and the fixed-combination agent. Twelve months postoperatively, the IOP remained controlled on the fixed combination alone, and his visual acuity was stable.
1. Yamane S, Sato S, Maruyama-Inoue M, Kadonosono K. Flanged intrascleral intraocular lens fixation with double-needle technique. Ophthalmology. 2017;124(8):1136-1142.
2. Watane A, Kalavar M, Pollmann AS, et al. Structural integrity of enVista MX60 eyelets. J Cataract Refract Surg. 2021;47(5):677.