

Coding regulations and policies can change several times a year or, during the COVID-19 pandemic, several times a week. The clear importance of timely access to new drugs and devices at this time has shone a spotlight on the Hospital Outpatient Prospective Payment System’s (OPPS) pass-through status.
Pass-Through Status
Pass-through status is awarded by the US Department of Health and Human Services on a case-by-case basis for newly FDA-approved drug and device products. Products qualifying for pass-through status include orphan drugs; drugs and biological agents used to treat cancer; certain new drugs; and, as clarified in the 2018 final OPPS 340B rule, biosimilar drugs.1,2
Section 1833(t)(6) of the Social Security Act allows payment for a product with pass-through status for at least 2 years but typically no longer than 3 years. For products with pass-through status that are used in a hospital setting, the CMS reimburses 100% of the cost for patients covered by Medicare Part B, and no copayment applies. When a drug or device with pass-through status is used in an ambulatory surgery center (ASC), however, the statutory 20% copayment applies, although it is typically covered by a patient’s supplemental insurance. For patients with Medicare Advantage or another commercial insurance plan, coverage and payment vary, and it is best to check with the individual carrier for guidance.
Each year, CMS estimates how much Medicare will spend on transitional pass-through devices, drugs, and biologics in the coming year and sets aside that amount to cover payments through the year. Essentially, the money is allocated upfront. Payment for pass-through drugs is set at the payment rate of average sales price + 6%, with the rates updated quarterly. The initial payment for the new device or drug is established based on a complex formula, which establishes a floor price above which the product must be priced. Reimbursement is then based on this price.
Drugs and Devices That Currently Have Pass-Through Status
Several ophthalmic drugs and devices currently have pass-through status, including but not limited to the following:
- Omidria (Omeros): used during cataract surgery and IOL replacement surgery to maintain pupil size by preventing intraoperative miosis and reduce postoperative ocular pain. Omidria was initially designated by the C-code C9447 but was given the permanent J-code J1097 effective October 1, 2019. In 2018, Omidria’s pass-through status was extended for 2 additional years, which expired in 2020.3 In December 2020, CMS confirmed that Omidria qualifies for a separate payment under the nonopioid pain management surgical drugs policy when used in the ASC setting for calendar year 2021.4
- Dexycu (EyePoint Pharmaceuticals): an intracameral steroid for the treatment of postoperative inflammation. Dexycu was initially designated by the C-code C9034 but is now reimbursed by the J-code J1095 effective January 1, 2019.
- Dextenza (Ocular Therapeutix): a corticosteroid used for the treatment of ocular inflammation and pain following ophthalmic surgery. Dextenza was issued the C-code C9048 effective July 1, 2019, but was granted the J-code J1096 effective October 1, 2019.
- CustomFlex ArtificialIris (HumanOptics): a custom-made prosthetic iris used to treat congenital and traumatic aniridia in addition to iris defects. The CustomFlex ArtificialIris was issued the C-code C1839 (iris prosthesis) effective January 1, 2020. Category 3 Current Procedural Terminology (CPT) codes 0616T, 0617T, and 0618T are used to report the device’s implantation effective July 1, 2020.
Speedbumps in Pass-Through Status
The goal of pass-through payments is to facilitate patient access to innovative devices and drugs, but there are a few speedbumps. After a drug or device’s pass-through status expires, it may be packaged and reimbursed as part of the facility fee for which the hospital or ASC would otherwise receive payment. This fee does not always cover the cost of the drug or device. ASCs operating on tight margins may be unable to provide patients with access to all FDA-approved medications with postoperative indications because they are too costly to include in the bundled facility fee. Further, when a drug’s pass-through status expires, its use often declines once its cost is bundled into the Ambulatory Payment Classification payment because of the ASC’s inability to afford the drug.
CMS has not developed a policy that provides separate Medicare Part B coverage and payment for drugs that are administered at the time of ophthalmic surgery and have FDA-approved indications for the treatment/prevention of postoperative issues. However, CMS does have a policy that allows separate payment for nonopioid pain management drugs, such as Omidria, that function as surgical supplies when furnished in the ASC setting. This policy excludes drugs meeting this definition from packaging under the ASC payment system.5 It is important that CMS revisit the issue of bundling drugs used in ophthalmic surgery where there is a postoperative (not a surgical) application aside from nonopioid pain management in order to provide fair reimbursement.
Healthcare Common Procedure Coding System and CPT Codes
When pass-through status is granted for a product, CMS designates a Healthcare Common Procedure Coding System (HCPCS) code, which must be used for billing and to obtain reimbursement. HCPCS codes allow CMS to collect claims data on cost and utilization levels, which are a component in CMS’ formula to determine facility payment once the pass-through status for a product expires.
The HCPCS code set and CPT code set are not synonymous. CMS developed HCPCS, and the American Medical Association developed CPT through the CPT Editorial Panel. HCPCS has three levels:
- Level 1 is the American Medical Association’s CPT code set;
- Level 2 consists of alphanumeric codes that include nonphysician services; and
- Level 3 includes local codes developed by state Medicaid agencies, Medicare contractors, and private insurers for use in specific programs and jurisdictions.
HCPCS level 1 codes are identical to CPT codes but are technically HCPCS codes when billed to Medicare or Medicaid. HCPCS level 2 codes are used to report products used during services performed outside a physician’s office and are reported in conjunction with CPT and ICD-10-CM codes.6
National Correct Coding Initiative
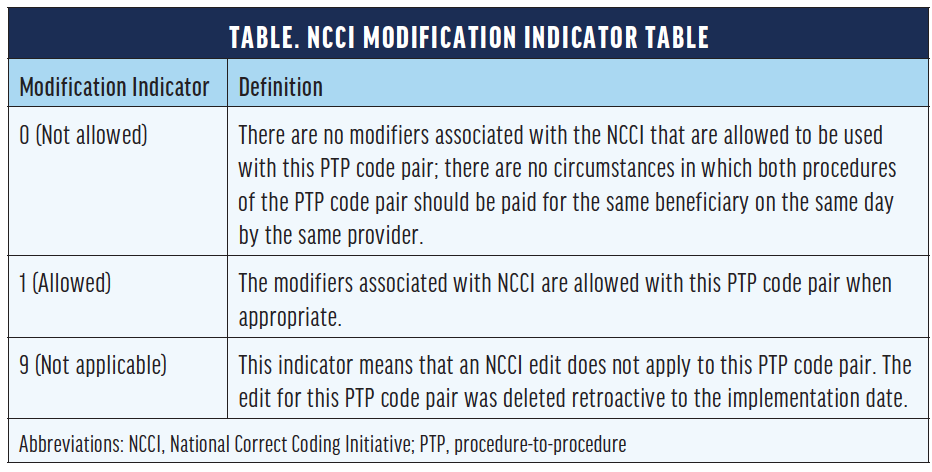
The National Correct Coding Initiative (NCCI), developed by CMS, designates a modification indicator for when a modifier can be used (Table). CPT 67028 Intravitreal injection of a pharmacologic agent (separate procedure) has a zero-day global period, meaning payment includes the procedure/service plus associated care provided on the same day of service. For an intravitreal injection, the use of modifier -25 with an evaluation and management (E/M) code is often, but not always, appropriate and is designated under indicator 1. Modifier -25 denotes a “significant, separately identifiable [E/M] service by the same physician or other qualified health care professional on the same day of the procedure or other service.” E/M services also include eye visit codes CPT 92002, 92004, 92012, and 92014. Both the NCCI and recommendations from the AAO indicate modifier -25 may be appended to an E/M service when provided on the same day as an intravitreal injection, with exceptions.7
CONCLUSION
J-codes, C-codes, and V-codes are encountered often in ophthalmology. J-codes J0120 through J8999 represent drugs administered by routes other than the oral method, C-codes C1713 to C9899 represent OPPS, and V-codes V2020 to V2799 are for vision services. J-codes are more advantageous than C-codes because J-codes are permanent codes that may be used across all government and third-party insurers nationwide. In contrast, C-codes are temporary and valid only for Medicare coverage of OPPS services and procedures claims.
1. Process and information required to determine eligibility of drugs, biologicals, and radiopharmaceuticals for transitional pass-through status under the hospital outpatient prospective payment system (OPPS). CMS. Modified January 2015. Accessed January 19, 2021. cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Downloads/drugapplication.pdf
2. Medicare program: hospital outpatient prospective payment and ambulatory surgical center payment systems and quality reporting programs. Gov info. Department of Health and Human Services Centers for Medicare & Medicaid Services. December 14, 2017. Accessed January 19, 2021. govinfo.gov/content/pkg/FR-2017-12-14/pdf/R1-2017-23932.pdf
3. Cataract-surgery drug gains temporary pass-through payment extension for ASC use. American Academy of Ophthalmology. March 29, 2018. Accessed January 19, 2021. https://www.aao.org/eye-on-advocacy-article/cataract-surgery-drug-gains-temporary-pass-through
4. The financial impact of the CMS final fee schedule rule on ophthalmic practice. American Academy of Ophthalmology. December 8, 2020. Accessed January 22, 2021. https://www.aao.org/practice-management/multimedia-detail/financial-impact-cms-final-fee-schedule-rule
5. Medicare program: hospital outpatient prospective payment and ambulatory surgical center payment systems and quality reporting programs. Department of Health and Human Services Centers for Medicare & Medicaid Services. November 23, 2020. Accessed January 22, 2021. https://www.cms.gov/files/document/12220-opps-final-rule-cms-1736-fc.pdf
6. All about HCPCS. AAPC. Accessed January 19, 2021. https://www.aapc.com/resources/medical-coding/hcpcs.aspx#:~:text=HCPCS%20Level%20I%20consists%20of,%2C%20laboratories%2C%20and%20outpatient%20facilities
7. Vicchrilli S, Williams GA. Modifier–25 revisited. American Academy of Ophthalmology. January 2021. Accessed January 19, 2021. https://www.aao.org/eyenet/article/modifier-25-revisited




