

Two major hurdles remain in the quest to optimize patients’ functional vision after cataract surgery: (1) the ability to adjust refractive power accurately and easily and (2) the ability to preserve or restore accommodation. Many other objectives exist, such as inhibiting lens epithelial cell proliferation, compensating for chromatic aberration without diffraction, and eliminating induced dysphotopsias. Overcoming the two major hurdles would be a significant achievement, especially if a wide range of IOL adjustments could be executed over a patient’s lifetime.
Many adjustable IOLs are in the research pipeline, and it is hoped that several will eventually reach the market. At present, the only IOL approved for postoperative power adjustment by the US FDA is the Light Adjustable Lens (LAL; RxSight). This unique three-piece silicone IOL has unpolymerized photosensitive macromers that can diffuse along a concentration gradient after UV light treatment. The LAL’s power adjustability is ±2.00 D of sphere and ±2.00 D of cylinder, ranges that encompass most patients’ postoperative refractive errors. The desired refractive outcome must be locked in after it has been achieved, after which the LAL ceases to be adjustable. RxSight is currently working on a two-photon chemistry that may permit continuous adjustability without requiring patients to wear UV-blocking glasses.
In a different approach to adjustability, Perfect Lens is developing technology known as refractive index shaping that modifies the chemistry of virtually any off-the-shelf one- or three-piece acrylic lens versus an IOL specifically manufactured by the company.
HOW REFRACTIVE INDEX SHAPING WORKS
The infrared femtosecond lasers used for conventional refractive and cataract surgery operate at high energy levels to create plasma, shock waves, and cavitation bubbles. In contrast, Perfect Lens’ laser operates at lower energy levels and can create nondisruptive chemical changes within materials such as hydrophobic and hydrophilic acrylic. This low-powered laser produces femtosecond pulses in a visible green wavelength with a precise spatial resolution to treat and change the refractive index of a small sliver of an acrylic IOL. Each pulse of energy creates hydroxy and acid groups within the acrylic material, which alters the hydrophilicity of that area of the optic. This, in turn, changes the local water content of the lens and, consequently, its refractive index.1 The alteration occurs without generating toxins that might leach out of the IOL.
Applying spatially precise laser treatments in a controlled manner over a thin sliver of the acrylic IOL allows a separate refractive or diffractive lens to be created within the IOL. Because each treatment area occupies only a thin slice of the anteroposterior dimension of an IOL, multiple treatments can theoretically be stacked over several sessions to make the IOL almost continuously adjustable. Changes that can currently be applied include alterations in sphere, cylinder, and asphericity and the addition and subtraction of multifocality (Figure 1). Wavefront-guided treatments may be possible in the future.
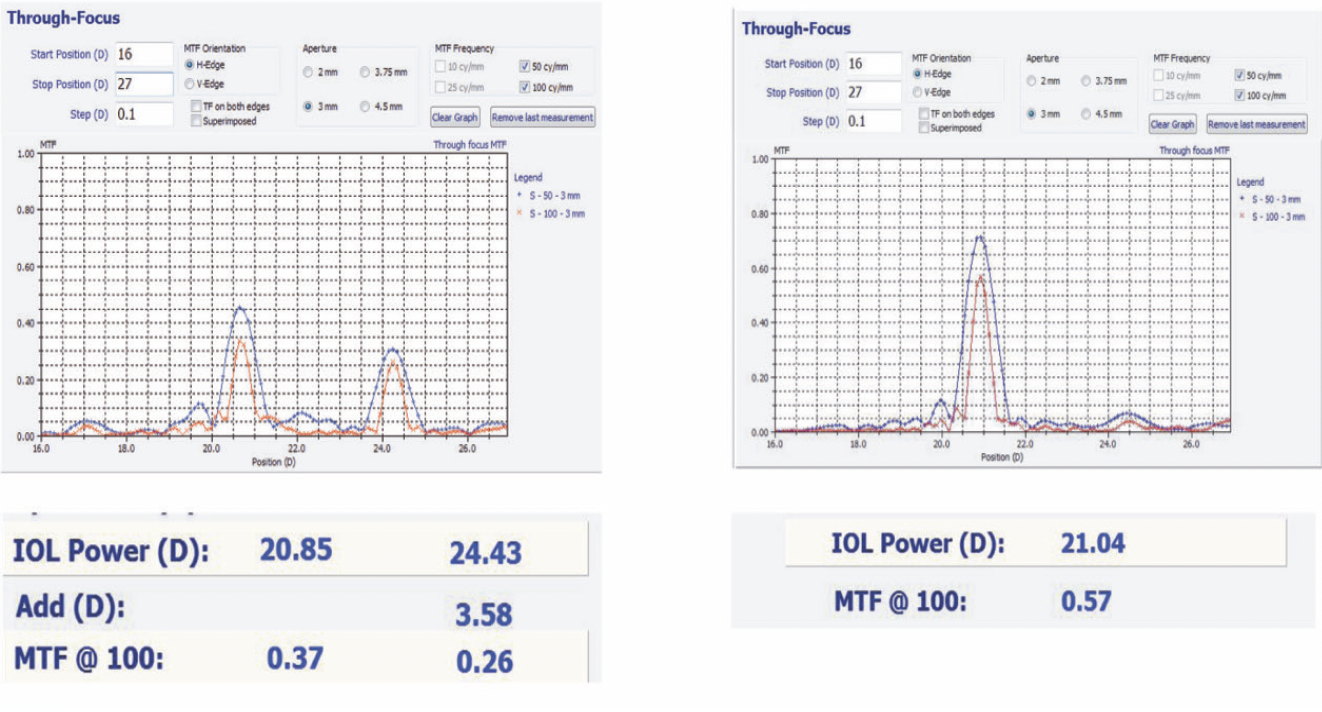
Figure 1. The optical bench through-focus curve of a multifocal IOL (left) was turned into a monofocal IOL (right) with Perfect Lens’ refractive index shaping technology. Abbreviation: MTF, modulation transfer function.
RESEARCH RESULTS
Rabbit Experiment
In an experiment conducted at the John A. Moran Eye Center at the University of Utah and published in 2017,2 preloaded CT Lucia 601PY IOLs (Carl Zeiss Meditec) were implanted into the eyes of six white, female, New Zealand rabbits. One eye of each rabbit was treated with the Perfect Lens laser, while the other eye served as a control. The refractive target was a +3.60 D change in IOL power. The laser procedure lasted 23 seconds. The animals were sacrificed 2 weeks after treatment, and the IOLs were removed for analysis.
The average change in power achieved in the six treated IOLs was 3.58 ±0.26 D. Neither toxicity nor excessive inflammation was found in any treated eye. The only adverse event observed was that some of the treatments were mildly decentered.
Laboratory Experiment
In a study conducted at the Moran Eye Center and published in 2018,3 10 hydrated CT Lucia 601PY IOLs were subjected to laser treatment in an experimental test chamber. The goal was to achieve a change in IOL power of -2.00 D. The results showed a mean power shift of -2.037 ±0.047 D, with a corresponding mean change in modulation transfer function of -0.064 ±0.053 As anticipated, there was a slight decrease in light transmittance and a slight increase in back scattering. These levels, however, were not expected to be clinically significant.
First Experiments in Humans
In 2018, the first human treatments were administered to two patients in Panama. These initial experiments provided valuable insights into the process of docking the laser and performing the treatment (unpublished data).
A formal, nonrandomized, prospective study was initiated at the same practice in 2019. All six patients in this study received a Tecnis Monofocal 1-piece hydrophobic acrylic IOL (Johnson & Johnson Vision). Postoperative laser treatments were conducted to correct spherical, cylindrical, and spherocylindrical errors. The trial was paused during the COVID-19 pandemic, and several original patients were lost to follow-up. When the study resumed in 2022, hardware and software upgrades had been implemented (Figure 2). Another six patients received treatment, with an average duration of 89 seconds. The primary efficacy endpoint of the second phase was a reduction in manifest refraction spherical equivalent refractive error at 7, 30, and 90 days after treatment. The primary safety endpoint was device-related adverse events (post- vs pretreatment).

Figure 2. The femtosecond laser treatment apparatus used in Panama.
A statistically significant (P = .0202) reduction in manifest refraction spherical equivalent refractive error was observed among the treated patients. None experienced a decrease in corrected distance visual acuity (P = .7231). Additionally, their mesopic contrast sensitivity, with and without glare, improved on average after the treatment. A visual function questionnaire found that patients who had difficulty with their uncorrected distance visual acuity before the study reported improvements. Similarly, those who felt as if they were “looking through a dirty glass” and those who experienced fluctuations in vision before the laser treatment noted improvements after treatment. Due to the pandemic, not all patients attended every postoperative visit. Nonetheless, the study identified areas for future improvement. Patient interface design changes were identified. Reducing treatment time would also be beneficial for patient comfort and clinical efficiency.
NEXT STEPS
The next step in the Perfect Lens journey involves a clinical trial in Zlín, Czech Republic, with plans for potential expansion. The goal is to obtain initial CE Mark approval for the laser, which would pave the way for seeking US FDA approval. This European clinical trial will include the creation of a multifocal lens within a monofocal IOL.
1. Bille JF, Engelhardt J, Volpp HR, et al. Chemical basis for alteration of an intraocular lens using a femtosecond laser. Biomed Opt Express. 2017;8(3):1390-1404.
2. Werner L, Ludlow J, Nguyen J, et al. Biocompatibility of intraocular lens power adjustment using a femtosecond laser in a rabbit model. J Cataract Refract Surg. 2017;43(8):1100-1106.
3. Nguyen J, Werner L, Ludlow J, et al. Intraocular lens power adjustment by a femtosecond laser: In vitro evaluation of power change, modulation transfer function, light transmission, and light scattering in a blue light-filtering lens. J Cataract Refract Surg. 2018;44(2):226-230.




