Adaptive Vision Through a Fluid-Based Design
By Sumit “Sam” Garg, MD

The Juvene IOL (LensGen) is a novel, modular, two-piece silicone IOL consisting of base and fluid lenses. The base lens has a circumferential blue haptic that supports a clear central optic. The fluid lens includes a clear posterior optic and a flexible anterior surface. It is filled with proprietary silicone oil. The fluid lens fits into the base lens under three haptic tabs, completing the Juvene IOL system.
MECHANISM AND DESIGN BENEFITS
Mechanism of Action
The Juvene IOL’s mechanism of action leverages natural capsular bag forces transferred to the base lens haptic, compressing the fluid lens and changing its anterior curvature (Figure 1). This results in a power change with near viewing effort and provides near and intermediate vision. Although the precise mechanism is still under investigation, laboratory and initial clinical results support the anterior curvature-changing dynamic. Previous in vivo studies have shown that the evacuated capsular bag can transfer forces during accommodative effort, lending credibility to the Juvene IOL’s proposed mechanism of action.
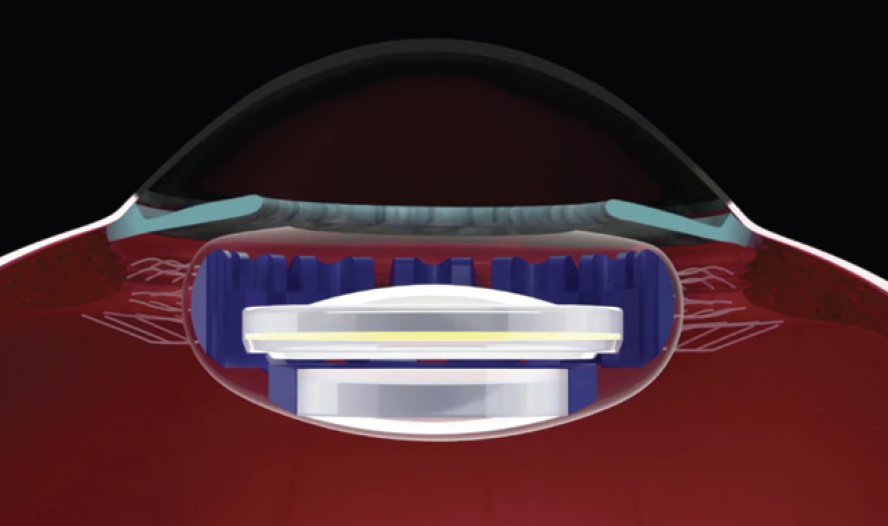
Figure 1. A schematic of the Juvene IOL demonstrates the shape-changing anterior optic of the fluid lens.
Figures 1 and 2 courtesy of Sumit “Sam” Garg, MD
Modular Design and Benefits
The Juvene IOL’s modular design theoretically facilitates future fluid lens exchange and potential upgradeability. The base lens can remain within the capsular bag, and the fluid lens can be exchanged. With its nondiffractive optic design, the Juvene IOL provides patients with quality of vision similar to that achieved with a monofocal lens and the extended visual range typical of diffractive IOLs.
CLINICAL DATA
Data on the Juvene IOL’s efficacy and safety over a 36-month period were presented at the 2023 ASCRS Annual Meeting.1 Monocularly, patients’ mean corrected distance visual acuity was 20/18, their corrected intermediate visual acuity was 20/26, and their corrected near visual acuity was 20/35. Further improvements were observed with binocular summation. Of the 18 eyes evaluated, including 10 eyes of five patients with bilateral Juvene IOLs, the mean distance-corrected monocular defocus curve remained above 20/40 from approximately +1.50 to -2.00 D. This indicates a roughly 3.50 D accommodative range of usable vision.
The 36-month monocular and binocular outcomes were comparable to or better than the 12- and 24-month results, although many patients were lost to follow-up. The mean manifest refractions remained stable over 36 months, demonstrating durable refractive and accommodative outcomes. This highlights the effectiveness of the Juvene IOL’s bag-filling design, which has shown low rates of posterior capsular opacification (PCO) and good stability in both clinical and animal studies. Mesopic contrast sensitivity was similar to that with a monofocal IOL. Additionally, there were no safety issues or PCO, and the amount of endothelial cell loss was comparable to that seen with conventional cataract surgery.
Patients reported excellent contrast sensitivity and minimal dysphotopsias on a directed questionnaire.
SURGICAL PROCEDURE AND PATIENT SELECTION
The lens can be inserted through a 3-mm incision with minimal alteration to the standard cataract surgical procedure (Figure 2). The implant’s modular feature theoretically facilitates an exchange if needed and allows an upgrade in the future.
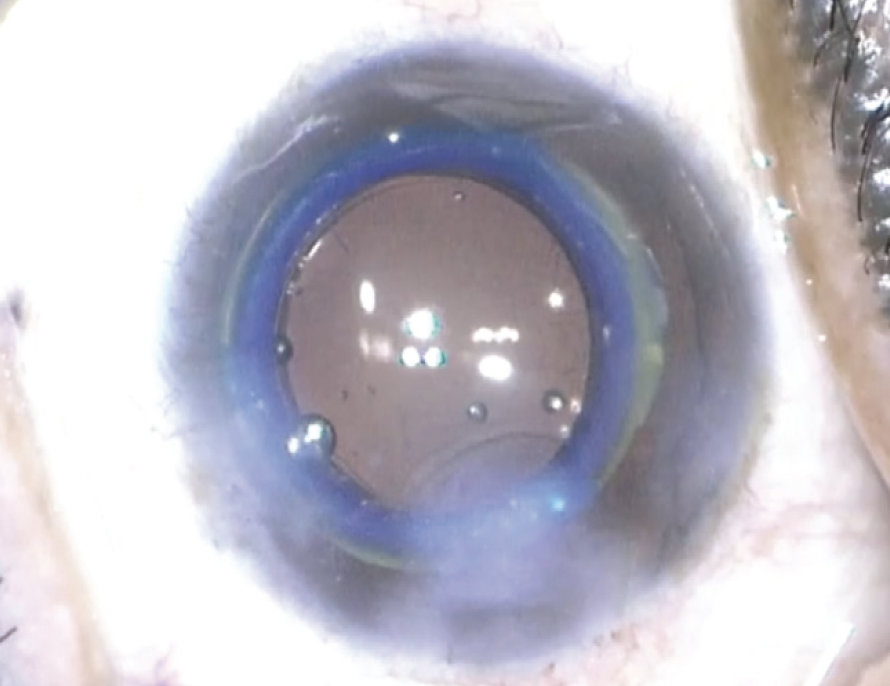
Figure 2. Intraoperative photograph of the Juvene IOL after implantation.
Preoperative counseling should be simpler with the Juvene IOL compared to currently available presbyopia-correcting diffractive IOLs. The biomimetic mechanism of action is fairly easy to understand. Additionally, many of the contraindications for currently available presbyopia-correcting IOLs do not apply to the Juvene IOL, because it provides monofocal-like optical quality without the contraindications typical of diffractive IOLs.
CONCLUSION
The 3-year data on the Juvene IOL indicate that it is a highly effective and safe option for patients with presbyopia and cataracts. The lens has provided excellent visual acuity across a range of distances, demonstrated a strong safety profile, and achieved high patient satisfaction.
Mimicking Pediatric Lens Accommodation
By John Vukich, MD

The primary objective of any accommodating IOL is to restore the eye’s natural ability to change focus similarly to a child’s native lens (Figure 3). The JelliSee Accommodating IOL (JelliSee Ophthalmics) is specifically designed to mimic this functionality.
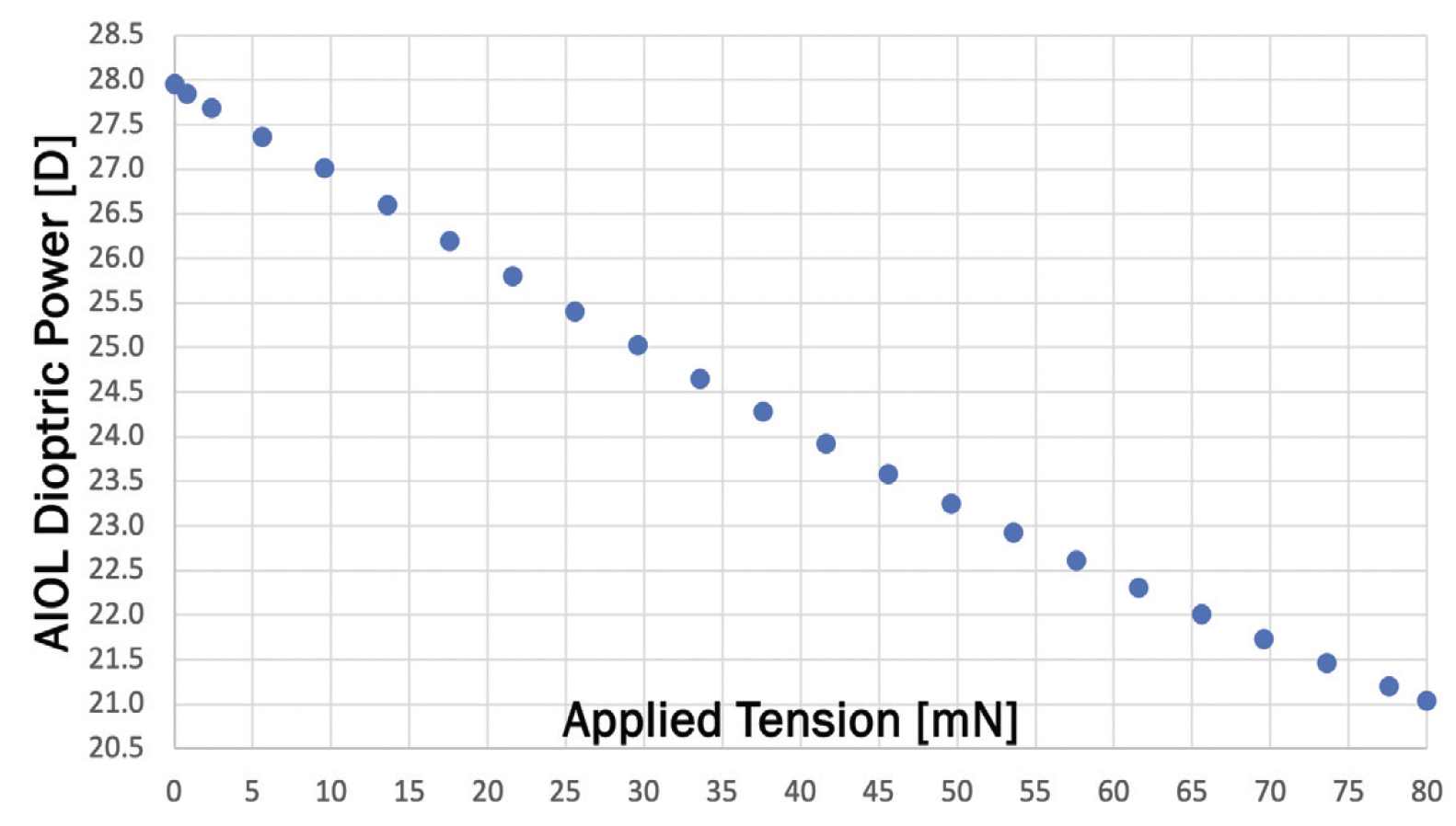
Figure 3. The dioptric power of the JelliSee lens changes linearly with applied tension using known forces within the eye.
Figures 3-5 courtesy of John A. Vukich, MD
PEDIATRIC HUMAN LENS MODEL
A human pediatric lens, in its relaxed state, is naturally accommodated. When the lens is removed from the eye, the zonular fibers are cut, and it is placed in neutral media, the lens surfaces become more curved, and the dioptric power increases. In children, the lens capsule is significantly stiffer than the softer lens cortex and nucleus.2 Conceptually, the lens capsule doesn’t squeeze the lens during accommodation but flattens the lens into disaccommodation.
SELECTIVE ZONULAR FORCE
Accommodation occurs when the ciliary muscle contracts, reducing the tension on the zonules, which makes the surface curvature of the lens more convex (rounded), resulting in increased lens power and improved near focus. Disaccommodation happens when the ciliary muscle relaxes, increasing zonular tension, which flattens the lens and reduces its power for distance vision.3 Interestingly, most of the dioptric power change during accommodation is due to the change in curvature of the anterior lens surface.4 Functionally, the anterior zonular fibers change the lens’ power during accommodation, while posterior zonules maintain its stability in the z-axis.5
ACCOMMODATIVE NEEDS
Typically, no more than 50% of the total amplitude of accommodation is used for sustained near focus; otherwise, asthenopia and fatigue occur.6-9 For example, a 20-year-old with an accommodative amplitude of 10.00 D can comfortably maintain 5.00 D of accommodation. By 50 years of age, presbyopia reduces the amplitude of accommodation to about 2.50 D, with only 1.25 D of sustained accommodation available, which is insufficient for prolonged near work.
Reading glasses, contact lenses, and multifocal implants typically provide +2.50 D of near add when used for presbyopia correction. This provides all required power without utilizing the ciliary muscle. Since only 50% of total accommodation may be used for sustained near work when the ciliary muscle is utilized, an accommodating IOL with at least 5.00 D (2 x 2.50 D) of accommodation is necessary for sustained near work.
DESIGN PARAMETERS OF THE JELLISEE IOL
Ideally, an accommodating IOL should achieve 5.00 D or more amplitude of accommodation. The lens should function independently of capsular fibrosis and require a change in diameter of less than 0.2 mm to achieve the full range of accommodation.3,10-12
There is evidence that 0.08 N of radial force is generated by the ciliary muscle, which is maintained in patients at least up to 80 years of age.3,10-18
The JelliSee IOL has a relatively flat anterior surface (similar to the natural lens) and a relatively firm anterior surface (similar to the pediatric lens capsule). The footplates, called actuators, are positioned into the peripheral capsular fornix, allowing the peripheral capsule to fibrose around them.19 The arm extending from the actuator attaches to the anterior surface of the IOL (mimicking selective zonular force). The anterior surface is universal for all powers of the IOL. The posterior optical element of the JelliSee IOL comes in a variety of spherical and toric powers (+6.00 to +34.00 D spherical equivalent; up to +2.75 D of cylinder), allowing the IOL to maintain the full amplitude of accommodation across the entire range of IOL powers.
The total thickness of the JelliSee IOL is 1.4 mm. Proof of concept has been achieved for insertion of the lens through a 3.4-mm incision, and efforts to reduce this size are encouraging. The lens has been independently validated with computer and optical modeling, laboratory bench testing, primate models, and now in humans.
VALIDATION AND PERFORMANCE
Finite element analysis, Zemax (OpticStudio) modeling, and optical bench testing demonstrate a linear change in spherical dioptric power of the JelliSee IOL with applied tension (Figure 3). This analysis predicts no discernible optical aberrations across the entire range of accommodation. Even with half the typically available zonular force, the JelliSee IOL provides 4.00 D or more of accommodation, meaning that individuals with suboptimal accommodation may still achieve sufficient accommodation with this implant. Dual pinhole optical bench analysis of a JelliSee IOL has confirmed excellent image quality across the entire range of accommodation, equivalent to the distance image quality of commercially available aspheric monofocal lenses.
In a primate model, 7.00 D of accommodation, measured pharmacologically with topical atropine (disaccommodation) and intravenous pilocarpine (accommodation), was achieved and maintained through 15 months of postoperative follow-up. Interestingly, the amplitude of accommodation increased from 4.00 D at 1 month to 7.00 D at 15 months as the capsule fibrosed.
As a primary investigator during the Quest JelliSee IOL study, I have performed all human surgeries to date. Figure 4 shows one of my first patients with the lens implant; the flat anterior accommodating surface, the thicker posterior power surface, and fluid-filled design are evident. Figure 5 demonstrates the monocular defocus curve of a JelliSee patient (70 years old, 1 year postoperatively) compared to a defocus curve of a healthy 10-year-old.
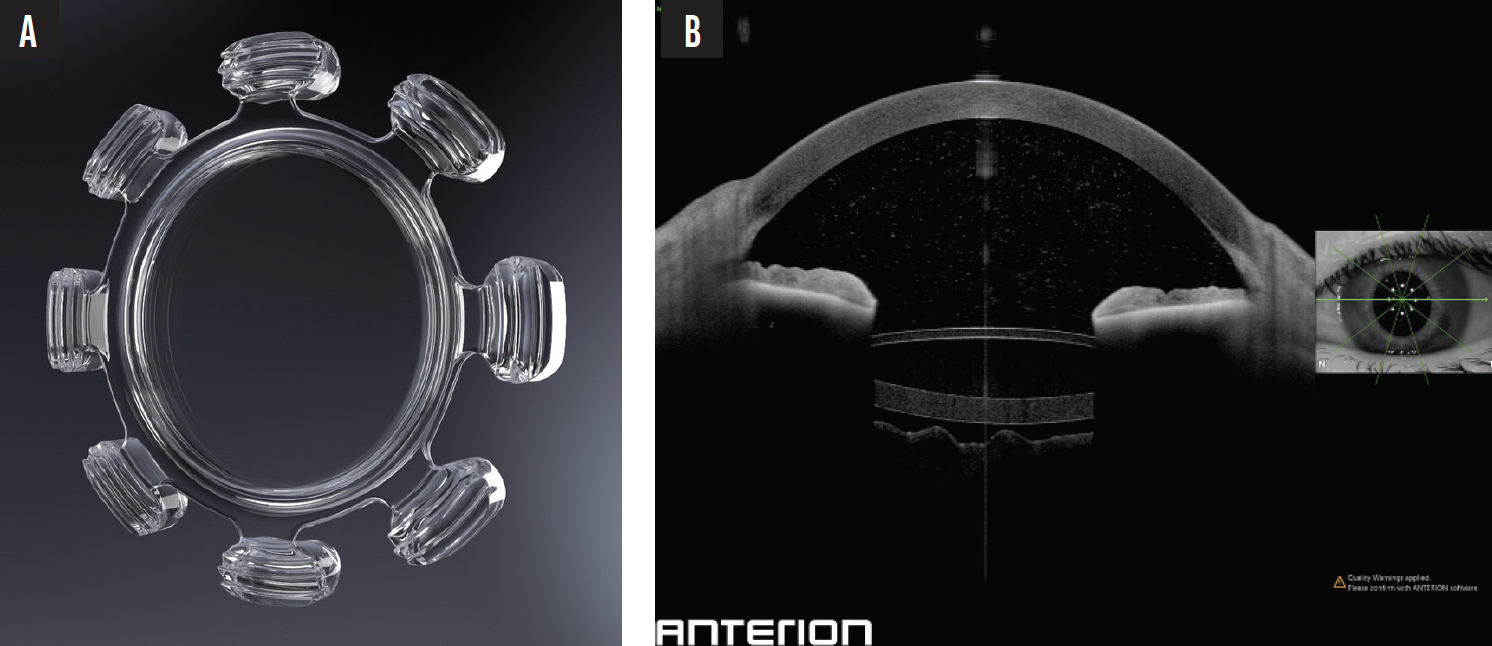
Figure 4. JelliSee IOL (A). An OCT scan of the IOL inside a human eye confirms the position and structure of the lens (B).
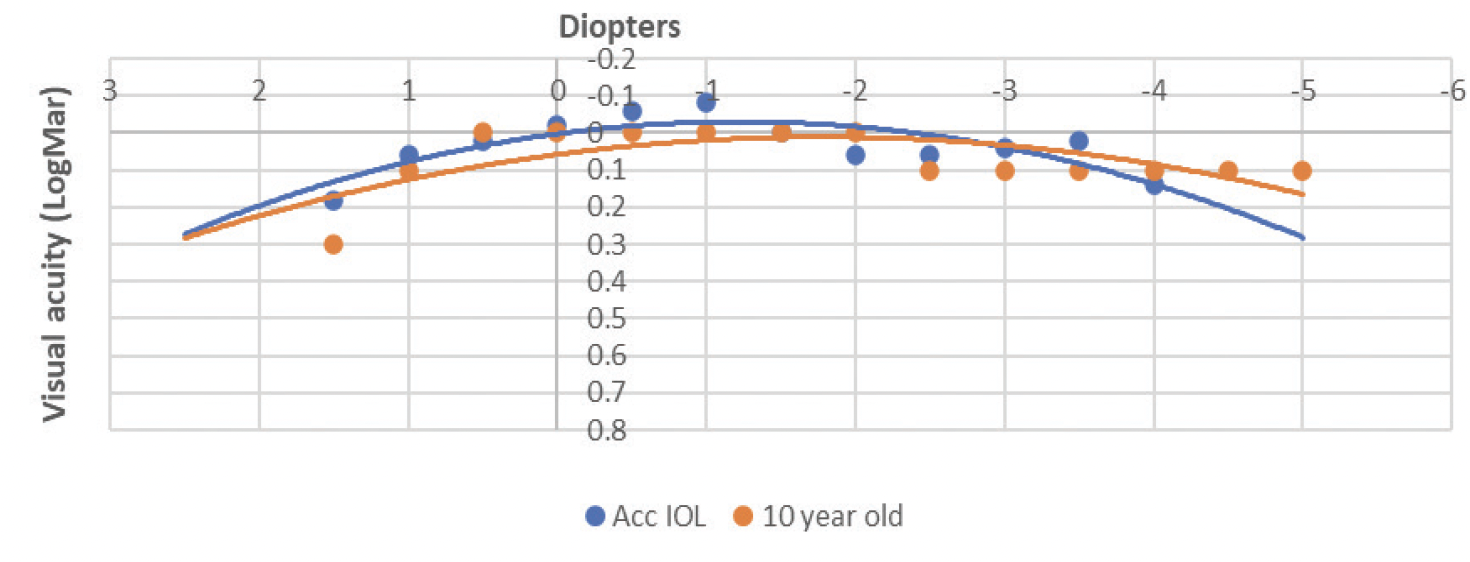
Figure 5. The monocular defocus curve of a 70-year-old JelliSee patient (1 year postoperatively) compared to a defocus curve of a healthy 10-year-old child.
NEXT STEPS
There are proof-of-concept human data demonstrating that the JelliSee IOL functions as intended. The implant is designed to mimic the pediatric human lens. Rotational stability data and accommodative amplitude data support the efficacy of the design. The next phase trial with up to 100 patients is scheduled to begin this fall/winter.
Shape-Changing, Modular Technology: Customizable and Adjustable
By George O. Waring IV, MD, FACS

The pipeline for shape-changing, modular IOLs is rapidly evolving through various approaches. Some lenses offer exchangeable optics, others use fluid-filled chambers and/or responsive materials that change shape in response to the accommodative mechanisms of the eye, and still others may use a combination of these mechanisms. Implants can be designed to mimic the eye’s natural accommodative ability to provide a full range of focus. Customization may be achieved through a variety of means after implantation. Soon, we surgeons may be able to fully address our lens replacement and cataract patients’ changing visual needs over time.
TWO-PIECE SYSTEM
The OmniVu (Atia Vision) is a modular IOL system consisting of two primary components: (1) a hydrophobic acrylic fixed-power front optic with docking tabs spaced at equal distances around the periphery and (2) a shape-changing biocompatible silicone base filled with silicone oil (Figure 6). The front optic docks into the base’s inner channel with the docking tabs.

Figure 6. Ultrasound biomicroscopy of the OmniVu IOL.
Figure 6 courtesy of George O. Waring IV, MD, FACS
The lens system is designed to restore a full range of vision binocularly with continuous through focus and a visual profile comparable to that of a high-quality monofocal IOL. The fluid-filled, shape-changing base is designed to simulate the natural accommodative mechanisms of the eye, which, as Daniel B. Goldberg, MD, has proposed, are more complex than previously thought.20 The force exerted by the ciliary muscles on the capsular bag translates to the shape-changing base, changing the thickness and curvature of the base optic and thereby increasing its power.
The lens is currently being studied outside the United States in patients undergoing cataract surgery.21 The customary inclusion and exclusion criteria for most IOLs are being used. After standard phacoemulsification, the base lens is inserted through a 3.5-mm incision into the capsular bag, through a capsulorhexis with an intended diameter of 5.0 mm. The front optic is injected separately into the capsular bag and then docked into the base. Careful attention should be paid to the capsulorhexis diameter to ensure uniform capsule-optic overlap.
DATA TO DATE
I presented the first-in-human 12-month data (25 eyes of 17 patients) at the 2024 ASCRS Annual Meeting22 and will present 24-month data at the upcoming AAO Annual Meeting in October. Patients achieved the postoperative target refraction, which remained stable out to 12 months. At 1 year, patients’ average uncorrected distance visual acuity was 20/16, their uncorrected intermediate visual acuity was 20/20, and their uncorrected near visual acuity was 20/25.
The binocular defocus curve remained 20/32 or better over 4.20 D (including the positive range of the defocus curve) with 5.00 D of continuous defocus at 20/40. Just as importantly, the OmniVu visual quality profile showed good contrast sensitivity performance. There were no unexpected adverse effects, and only one eye has required an Nd:YAG laser capsulotomy treatment to date. The capsulotomy procedure to treat PCO was performed successfully without any sequelae.
Interestingly, patients’ visual acuity continued to improve over time, raising promising considerations regarding effective lens position (ELP). Further, there was minimal to no PCO, likely because the anterior and posterior capsular leaflets were fully separated. I hypothesize that this may also result in unique biomechanical advantages for the lens’ method of action over time.
DISCUSSION
The accommodating IOLs in the pipeline share a common goal, but each attempts to restore the natural mechanism of accommodation in different ways. The OmniVu lens system, for instance, places its shape-changing component posteriorly instead of anteriorly. The more readily accessible anterior optic can be removed and replaced if the patient desires or needs a different optical situation. This design and location may have some advantages in terms of the optical power distribution of the shape-changing base.
In its current form, the OmniVu’s front optic is offered in a monofocal design, but I anticipate that toric models followed by other advanced optical designs and features will become available in the future.
Refractive IOLs are the fastest-growing segment of our industry. As sophisticated as intraocular surgery is in 2024, there are still a few unmet challenges. First, we cannot predict ELP over time, because we have no way of knowing how a given individual will heal. Second, we cannot control lens epithelial cell proliferation, which is directly related to ELP. Lastly, there is extensive variation in crystalline lens dimensions. My colleagues and I published the first normative database on physiologic lens volume. The average was 230.4 mm3, and there was a very wide range of 119.9 to 312.4 mm3 in lens volume.23 Currently, however, only one lens size is available.
I believe the OmniVu’s shape-changing modular technology offers a more physiologic approach than currently available IOLs. Potential platform-based benefits include stabilizing the ELP over time and reducing or eliminating PCO. The technology may even stabilize the zonules as the patient ages. The lens may also confer posterior segment advantages, such as stabilization of the anterior hyaloid membrane, which could help stabilize the vitreous and retina.
THE ROAD AHEAD
The OmniVu lens system is a potential solution to addressing patients’ visual needs over time. Additional opportunities for modular capsule-filling IOLs could include drug-eluting capabilities, IOP monitoring, virtual reality, and informatic sensors. The possibilities are exciting, but it all starts with the core platform concept, safety, and efficacy. The first-in-human trials for the OmniVu IOL system are promising, and I am optimistic and excited about the results so far.
1. Garg S, Donnenfeld ED, Devgan U, et al. Thirty-six-month visual outcomes after implantation of a modular, shape-changing, fluid-optic intraocular lens. Paper presented at: ASCRS Annual Meeting; May 6, 2023; San Diego, CA.
2. Danysh BP, Duncan MK. The lens capsule. Exp Eye Res. 2009;88(2):151-164.
3. Fisher RF. The ciliary body in accommodation. Trans Ophthalmol Soc U K (1962). 1986;105 (pt 2):208-219.
4. Dubbelman M, Van der Heijde GL, Weeber HA. Change in shape of the aging human crystalline lens with accommodation. Vision Res. 2005;45(1):117-132.
5. Nankivil D, Maceo Heilman B, Durkee H, et al. The zonules selectively alter the shape of the lens during accommodation based on the location of their anchorage points. Invest Ophthalmol Vis Sci. 2015;56(3):1751-1760.
6. Deepu S, Kujur ES, Horo S, Priyanka N, Selvin SST, Kuriakose T. Prescription of near addition and its relation to accommodative reserve in presbyopia – the dichotomy between theory and practice. Indian J Ophthalmol. 2021;69(7):1702-1706.
7. Grosvenor TP. Anomalies of refraction. In: Grosvenor TP, ed. Primary Care Optometry. 5th ed. Butterworth-Heinemann Elsevier; 2007:19-20.
8. Millodot M, Millodot S. Presbyopia correction and the accommodation in reserve. Ophthalmic Physiol Opt. 1989;9(2):126-132.
9. Rubin ML. Optics for Clinicians. 2nd ed. Triad Publishing; 1974:126.
10. Marchini G, Pedrotti E, Modesti M, Visentin S, Tosi R. Anterior segment changes during accommodation in eyes with a monofocal intraocular lens: high-frequency ultrasound study. J Cataract Refract Surg. 2008;34(6):949-956.
11. Strenk SA, Strenk LM, Guo S. Magnetic resonance imaging of aging, accommodating, phakic, and pseudophakic ciliary muscle diameters. J Cataract Refract Surg. 2006;32(11):1792-1798.
12. Modesti M, Pasqualitto G, Appolloni R, Pecorella I, Sourdille P. Preoperative and postoperative size and movements of the lens capsular bag: ultrasound biomicroscopy analysis. J Cataract Refract Surg. 2011;37(10):1775-1784.
13. Tabernero J, Chirre E, Hervella L, Prieto P, Artal P. The accommodative ciliary muscle function is preserved in older humans. Sci Rep. 2016;6:25551.
14. Weale RA. Presbyopia. Br J Ophthalmol. 1962;46(11):660-668.
15. Hermans EA, Dubbelman M, van der Heijde GL, Heethaar RM. Change in the accommodative force on the lens of the human eye with age. Vision Res. 2008;48(1):119-126.
16. Hermans EA, Dubbelman M, van der Heijde GL, Heethaar RM. Estimating the external force acting on the human eye lens during accommodation by finite element modelling. Vision Res. 2006;46(21):3642-3365.
17. Augusteyn RC, Mohamed A, Nankivil D, et al. Age-dependence of the optomechanical responses of ex vivo human lenses from India and the USA, and the force required to produce these in a lens stretcher: the similarity to in vivo disaccommodation. Vision Res. 2011;51(14):1667-1678.
18. Ziebarth NM, Borja D, Arrieta E, et al. Role of the lens capsule on the mechanical accommodative response in a lens stretcher. Invest Ophthalmol Vis Sci. 2008;49(10):4490-4496.
19. Hayashi H, Hayashi K, Nakao F, Hayashi F. Elapsed time for capsular apposition to intraocular lens after cataract surgery. Ophthalmology. 2002;109(8):1427-1431.
20. Goldberg DB. Computer-animated model of accommodation and theory of reciprocal zonular action. Clin Ophthalmol. 2011;5:1559-1566.
21. ClinicalTrials.gov. AVL200 IOL for treatment of cataract and presbyopia. Identifier NCT05627700. Updated July 28, 2023. Accessed July 2, 2024. https://clinicaltrials.gov/ct2/show/NCT05627700
22. Waring GO, Agarwal A, Chang D, et al. 12-month safety and effectiveness outcomes of a novel, modular, shape-changing, intraocular lens system. Paper presented at: 2024 ASCRS Annual Meeting; April 6, 2024; Boston, MA.
23. Waring GO 4th, Chang DH, Rocha KM, Gouvea L, Penatti R. Correlation of intraoperative optical coherence tomography of crystalline lens diameter, thickness, and volume with biometry and age. Am J Ophthalmol. 2021;225:147-156.




