CASE PRESENTATION
A 36-year-old White man presents for a refractive surgery evaluation. The patient is a computer programmer by trade who is in the US Air Force Reserve. He routinely wears toric soft contact lenses but discontinued wearing the lenses approximately 4 weeks before this visit. His primary goal is to reduce his dependence on glasses and contact lenses.
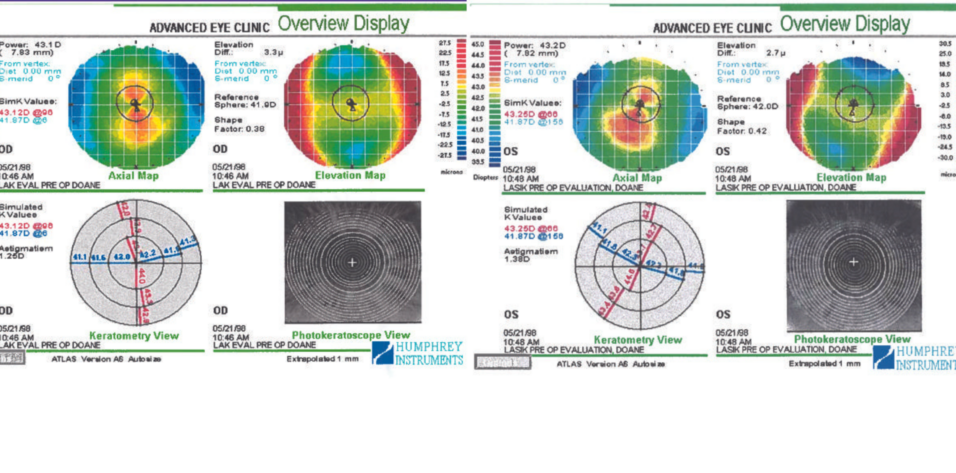
The patient’s medical history is significant for seasonal allergies, for which he administers a sinus decongestant and antihistamine pills as needed. His BCVA is 20/20 OU with a refraction of -6.75 +1.00 x 107º OD and -7.25 +2.00 x 052º OS. Figures 1 and 2 show his topographic measurements. Ultrasound pachymetry readings are 515 µm OD and 517 µm OS.
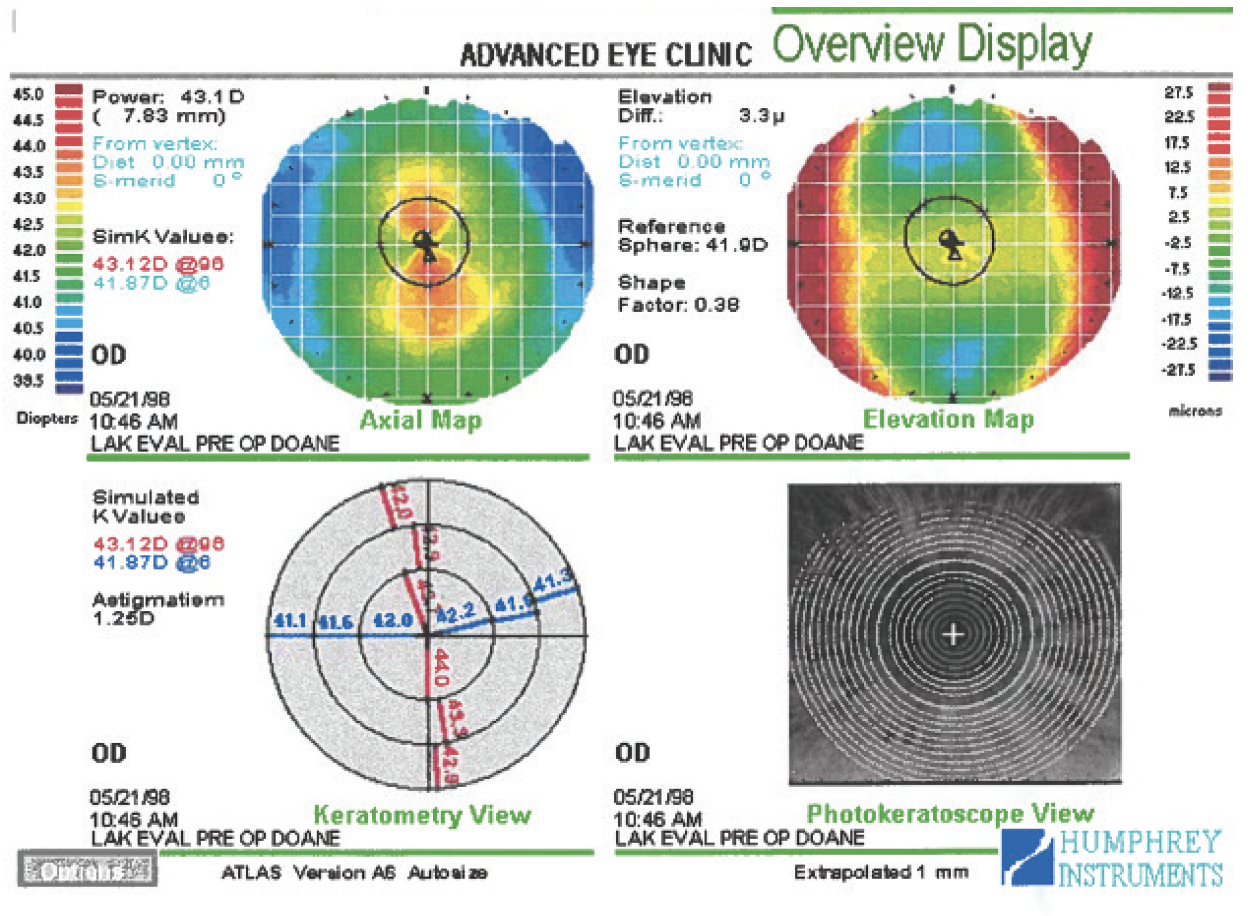
Figure 1. Preoperative topography of the right eye.

Figure 2. Preoperative topography of the left eye.
What would you recommend to the patient?
—Case prepared by John F. Doane, MD

RENATO AMBRÓSIO JR, MD, PHD, FWCRS
Front-surface Placido disc topography and central corneal thickness measurements are insufficient to comprehensively analyze an individual’s candidacy for laser vision correction (LVC). The patient’s moderate myopia and relatively thin central pachymetry readings demand a nuanced approach. Multimodal analysis is indispensable and extends beyond detecting potential mild keratoconus through front-surface topography to include a thorough assessment of ectasia susceptibility. The evaluation should incorporate tomographic and biomechanical assessments of the cornea. The application of AI, as proposed in the Tomographic and Biomechanical Index, should also be considered.1 Likewise, epithelial thickness measurements can be valuable when the topography is suspicious.2 The relational thickness alteration proposed by BrAIN (Brazilian Artificial Intelligence Networking in Medicine) offers an innovative approach to assessing the impact of surgery.
The patient’s refraction and corneal characteristics suggest a moderate risk of ectasia. I would therefore recommend a phakic IOL without comprehensive tomographic and biomechanical analysis. My thinking aligns with the principle of knowing ourselves and our adversaries or challenges, as espoused by Sun Tzu in The Art of War. In situations like this, understanding the intricate details of the patient’s corneal structure is essential, as is knowing ourselves and recognizing the relational weakening of the cornea based on the patient’s age and the limited thickness data available (ie, the challenge or adversary). Such an approach guides us toward a safer, more considered choice in refractive surgery.

J. BRADLEY RANDLEMAN, MD
The patient has high myopia, significant astigmatism, corneas that are thinner than average on ultrasound pachymetry, and suspicious anterior corneal curvature with focal central steepening in both eyes. The corneas are not steep overall despite their initial appearance (ie, presence of “hot” red colors centrally due to the use of a relative color scale). A truncated bowtie pattern is evident in the right eye, and greater inferior steepening is present in the left eye. The central keratometry (K) reading is approximately 42.00 D OU, so the predicted postoperative K reading would be approximately 35.00 to 36.00 D OU with any LVC procedure. The residual stromal bed thickness would be approximately 300 µm, but this is not reassuring given the asymmetry in anterior curvature.
The patient’s refractive error and age could make him a candidate for a phakic IOL, depending on anterior chamber depth and endothelial cell counts. If these are appropriate, a Visian Implantable Collamer Lens (ICL; STAAR Surgical) would be my first choice. I would not recommend any LVC procedure.

NANCY A. TANCHEL, MD
The patient could benefit from refractive correction. I would like to know if he is a pilot or plans to become one. A history of his myopia progression and astigmatism would also be helpful.
Topography shows symmetric astigmatism in the right eye and mildly asymmetric astigmatism in the left eye. The pachymetry readings are sufficient for a customized LASIK treatment in both eyes with a highly reliable, consistent femtosecond laser flap that is 110 µm thick. PRK would also be an option, depending on the patient’s lifestyle and whether he enjoys contact sports or has other reasons to prefer surface ablation.
He might have created his astigmatism by rubbing his eyes, which could be problematic. I would not treat him before obtaining more information regarding the overall corneal pachymetry. A view of the posterior cornea is also required to ensure no posterior bulging or forme fruste keratoconus is present, which is more likely in someone with allergies. I no longer perform refractive surgery on anyone if I cannot visualize the posterior cornea, but this would be especially important here.
THE CASE CONTINUED
The patient did not proceed with surgery because he could not obtain financing. He returns to the clinic 3 years and 3 months later still interested in surgery. Upon reevaluation, his BCVA is 20/20- OU with a refraction of -7.25 +2.00 x 110º OD and -6.75 +2.00 x 055º OS. Figures 3 and 4 show his topographic measurements. The ultrasound pachymetry measurements have remained stable since his earlier visit.
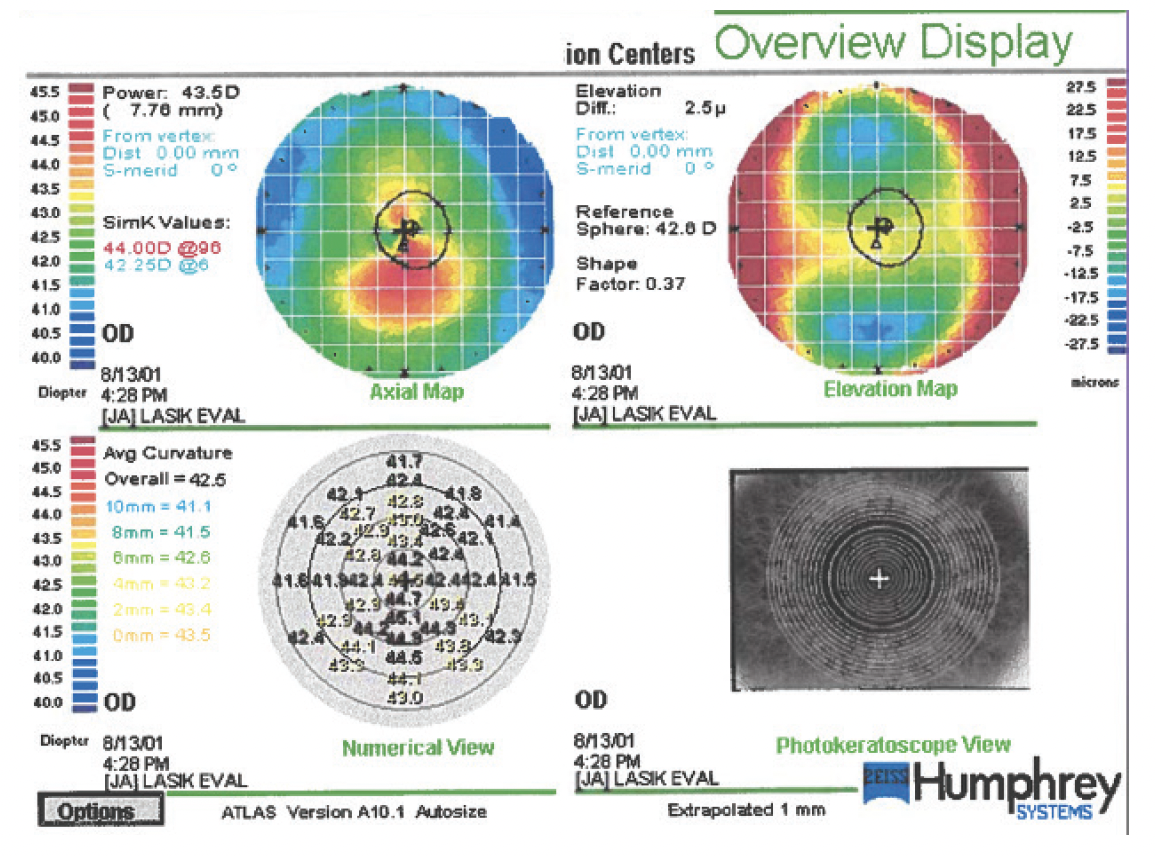
Figure 3. Topography of the unoperated cornea of the right eye 3 years after the measurements shown in Figure 1.

Figure 4. Topography of the unoperated cornea of the left eye 3 years after the measurements shown in Figure 2.
What would you recommend to the patient at this point?
DR. AMBRÓSIO
Based on the available data, I would still suggest a phakic IOL.
More information is required, but some observations are possible. There is some variation in the patient’s manifest refraction. Placido disc–based topography reveals steepening in both eyes. The left eye exhibits highly abnormal temporal flattening, which seems to be caused by ocular surface dryness or a scar. The Placido image reflected from the cornea of the left eye shows an irregular break in the reflex of the defocus ring at the temporal flattened zone. I recommend repeating the scans after the instillation of preservative-free artificial tears. Based on the clinical results, he might benefit from treatment to optimize the ocular surface before refractive surgery.
The central K readings increased in both eyes. The reference sphere for the elevation map increased significantly (> 1.00 D) in each eye. The reference rose from 42.60 to 44.00 D OD and from 42.00 to 43.30 D OS. This change would be detailed in subtraction (or differential) maps.
In the realm of medical decision-making, it behooves us to draw on the wisdom of Socrates, as described by his students Plato and Xenophon: “The only true wisdom is in knowing you know nothing” or “All I know is that I know nothing,” reminding us of the fundamental need to pursue knowledge. Socrates’ perspective is particularly relevant to elective refractive surgery. Embracing the Socratic method enhances the accuracy of medical diagnosis. It aligns with our ethical responsibility to provide the best possible care to patients under the founding principle of medicine attributed to Hippocrates: Primum non nocere or First, do no harm.
Multimodal refractive diagnostics should start with anamnesis. The chief complaint and clinical history are relevant. A better understanding of the patient’s dry eye symptoms and his contact lens and medical history is required. In addition to Placido disc–based topography, integrated tomography and biomechanical assessments with Scheimpflug imaging would help to characterize his ectasia risk.1,2 Segmental or layered tomography with epithelial thickness measurements using OCT or digital very high-frequency ultrasound are also relevant. I would exercise caution when evaluating the horizontal B-scans of the left eye.3 This may be a concept of optical biopsy, correlating with slit-lamp biomicroscopy, if there is a scar or subepithelial infiltrate, for example. Ocular wavefront analysis might help optimize the manifest refraction and provide an understanding of pupil size and other related metrics for the quality of vision. Anterior segment data obtained with OCT imaging and digital very high-frequency ultrasound should be considered when sizing a phakic IOL. Information on the axial length could help detect eye elongation.
Scientific evidence forms the foundation of medical guidelines. In addition to comprehensive patient education to make informed decisions, we must align with recommended practices. The concepts of must and should in diagnostics are relevant when evaluating refractive surgery candidates. Although ensuring safety is essential, wisdom from ancient philosophy empowers us to navigate the complex landscape of elective refractive surgery. Excellence benefits all involved parties. The call for a collaborative effort led by refractive surgeons and scientists is relevant for a critical and adaptable approach to technological advancements.
DR. RANDLEMAN
Since the patient’s initial presentation, the asymmetric curvature has worsened, with increased focal steepening in both eyes. The right eye now exhibits a classic asymmetric bowtie with skewed radial axis pattern, and the inferior cornea of the left eye has steepened by roughly 1.00 to 1.50 D inferiorly (slightly different scales and maps make it difficult to be more precise). His corrected distance visual acuity remains unchanged in both eyes. The refraction of the left eye has remained stable, but the cylinder in the right eye has increased by 1.00 D.
Although the topographic changes rule out LVC, the patient does not meet the strict criteria for progressive keratoconus. Given his age, I would recommend observation rather than CXL and follow up in 6 months with repeat imaging to ensure further progression has not occurred.
He remains a candidate for an ICL if the anterior chamber depth and endothelial cell count in each eye are adequate. Because of the change in topography and slight variation in refraction, however, I would delay surgery until 12 months of topographic and refractive stability can be demonstrated.
DR. TANCHEL
Measurements show relative stability in the left eye but increased astigmatism and somewhat greater topographic asymmetry in the right eye. I would like to know when topography was performed with respect to the patient’s contact lens wear. Were the images obtained right after or 2 weeks or longer after lens removal? Assuming the latter, I would have confidence that the maps are stable. Long-term contact lens wear can cause some anterior asymmetry, as seen on the maps, Without visualization of the posterior surface, I cannot determine whether this represents mild ectasia. A view of the posterior corneal surface would also allow me to assess the overall shape and strength of the tissue. Additionally, the maps of the left eye suggest dryness. Three years after his original assessment, a complete eye exam would also be necessary to assess any changes behind the cornea, which may change the therapeutic options.
Despite the slight increase in myopia and astigmatism in the right eye, I believe the patient would do well with PRK, although I would remind him to avoid rubbing the eyes. Excellent postoperative corneal hydration and allergy control would be a priority to prevent ocular itching.
At his age, his corneas are likely to be stable and unlikely to develop progressive ectasia, so I would not recommend concurrent PRK with CXL. It would be reasonable to discuss a monovision or mini-monovision strategy with him in anticipation of a reduction in his near visual acuity during the next several years.

WHAT I DID: JOHN F. DOANE, MD, FACS
At the second visit, despite the patient’s age, he had what appeared to be the onset of forme fruste keratoconus bilaterally. Upon examination, a faint suggestion of a posterior subcapsular cataract was discerned in each eye. He remained interested in surgical vision correction.
Over the next 4 years, the cataracts progressed to the point that the patient emphatically stated, “I want something done.” Two years after that, in 2007, his BCVA was 20/60 OD and 20/25- OS, which decreased to 20/400 OD and 20/80 OS with glare testing. The patient subsequently underwent uncomplicated bilateral cataract surgery.
His most recent topographic measurements were obtained in 2009 and are shown in Figures 5 and 6. At his most recent visit in 2023, his BSCVA was 20/15 and J1+ OU with a refraction of -2.25 + 3.75 x 115º OD and -2.50 + 1.75 x 040º OS.

Figure 5. Topography of the right (A) and left (B) eyes 11 years after the patient’s initial presentation and 2 years after uncomplicated cataract surgery with a spherical monofocal IOL.
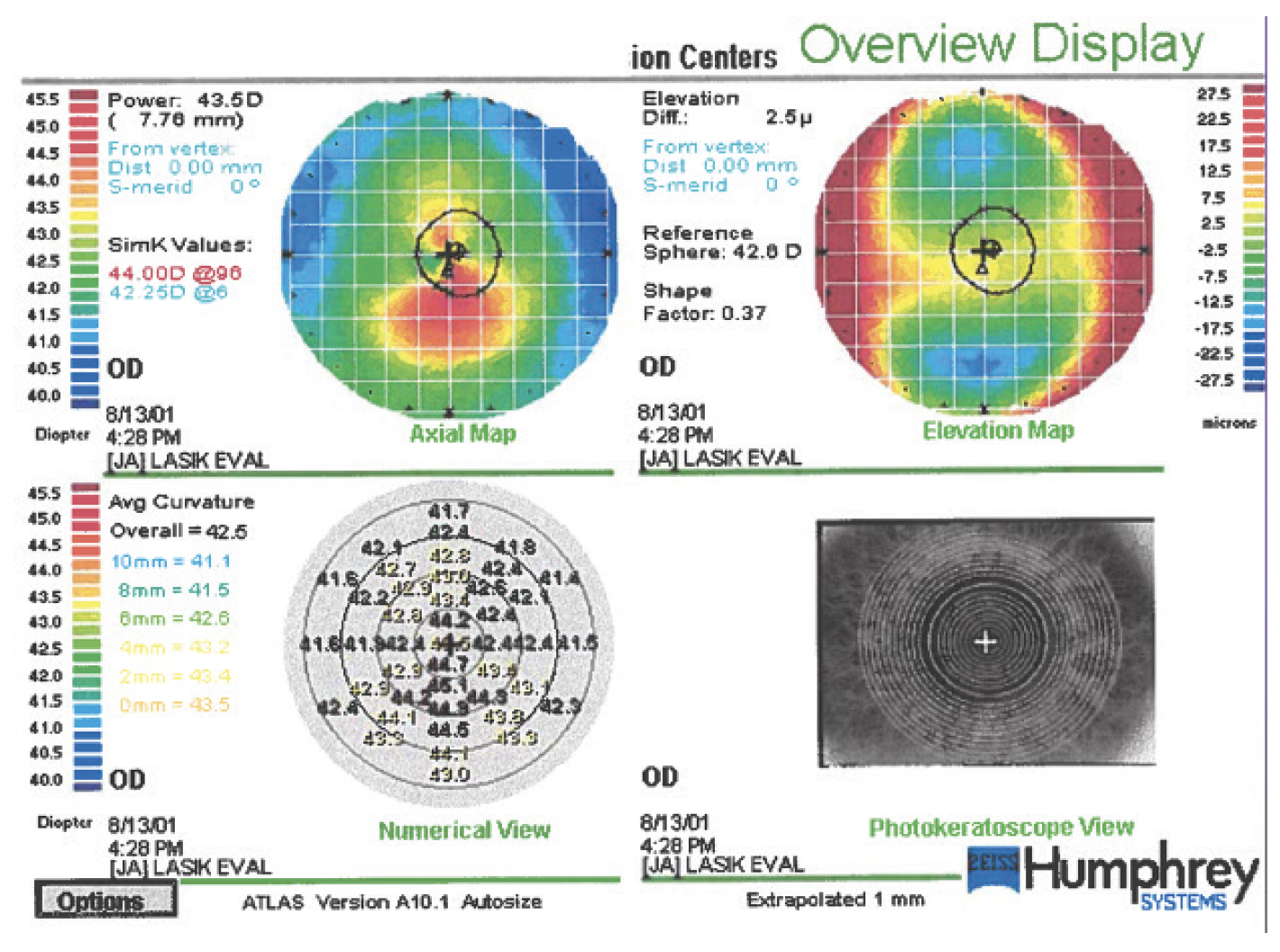
Figure 6. Left eye topography 11 years after the initial presentation and 2 years after uncomplicated cataract surgery with a spherical monofocal IOL.
Overall, the patient has been happy with his vision since undergoing cataract surgery. Had his second visit occurred today, CXL would certainly have been considered.
To me, the crux of this case is recognizing that spontaneous ectasia can occur, even in someone between the ages of 36 and 40 years. If this patient had undergone lamellar refractive surgery shortly after his first visit, he likely would have experienced rapidly progressive ectasia and lost lines of BSCVA, and the lamellar procedure would have been blamed for a condition that would have occurred without surgical intervention.
1. Ambrósio R Jr, Machado AP, Leão E, et al. Optimized artificial intelligence for enhanced ectasia detection using Scheimpflug-based corneal tomography and biomechanical data. Am J Ophthalmol. 2023;251:126-142.
2. Ambrósio R Jr, Salomão MQ, Barros L, et al. Multimodal diagnostics for keratoconus and ectatic corneal diseases: a paradigm shift. Eye Vis (Lond). 2023;10(1):45.
3. Salomão MQ, Hofling-Lima AL, Lopes BT, et al. Role of the corneal epithelium measurements in keratorefractive surgery. Curr Opin Ophthalmol. 2017;28(4):326-336.