

Cataract surgery has a good safety profile, and thanks to technological advances, there has been a steady decline in the rates of serious complications over the past few decades.1,2 As with any surgical procedure, however, perioperative difficulties can occur.
This article focuses on suprachoroidal hemorrhage, a rare but devastating complication. The reported incidence with cataract surgery over the past 25 years is 0.03% to 0.1%.1 For comparison, the incidence of suprachoroidal hemorrhage is 0.15% to 6.1% with glaucoma surgery, 0.17% to 1.9% with vitreoretinal surgery, and 0.09% to 1.08% with penetrating keratoplasty.1
MECHANISM
Consensus has not been reached on the mechanism of intra- and postoperative suprachoroidal hemorrhage. Common theories include a rupture of the short or long posterior ciliary artery, the vortex veins draining from the choroid during periods of hypotony, preexisting damage to the vessels that leads to choroidal effusion, subsequent stretching and rupture of the vessels, and ultimately bleeding into the suprachoroidal space.3-6
The choroidal vascular bed is responsible for the blood supply to photoreceptors in the retinal pigment epithelium and the optic nerve head. Disruption to this source of nourishment results in devastating vision loss.3,6
CLASSIFICATION BASED ON THREE PARAMETERS
Parameter No. 1: Relationship to intraocular surgery. When suprachoroidal hemorrhage develops during surgery, it is called acute intraoperative suprachoroidal hemorrhage (ASCH). When it develops during the postoperative period, it is called delayed suprachoroidal hemorrhage (DSCH).5
Parameter No. 2: Extent of hemorrhage. Suprachoroidal hemorrhage may be limited (involving one or two quadrants) or massive (involving three or four quadrants). With massive suprachoroidal hemorrhage, the hemorrhage may push the choroid and the inner retinal surfaces into direct apposition, usually within the center of the posterior chamber. This extensive hemorrhage is commonly referred to as kissing suprachoroidal hemorrhage (Figure).7
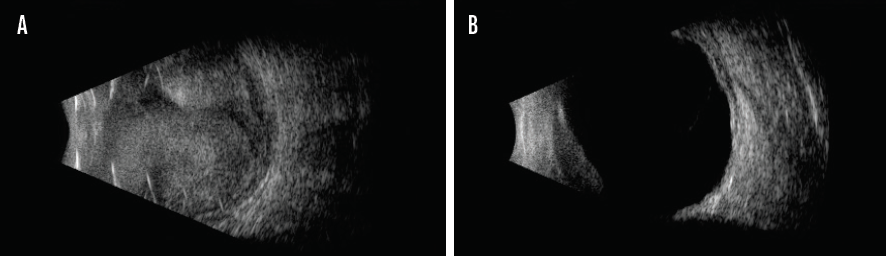
Figure. This eye exhibits 360º nonserous kissing choroidal detachment. The retina remains attached (A). Resolution of choroidal hemorrhage without retinal detachment 8 weeks after clinical presentation in the same eye (B).
Parameter No. 3: Precipitating events. Suprachoroidal hemorrhage may be spontaneous, caused by blunt or penetrating trauma, or perioperative.8
PREVENTION
A familiarity with risk factors can help surgeons to minimize the incidence of suprachoroidal hemorrhage.9-11
Patient-related. Nonmodifiable patient-related risk factors include advanced age, White race, medical comorbidities (cardiovascular disease, diabetes, hypertension, peripheral arterial disease), ocular comorbidities (glaucoma, high myopia, aphakia, pseudophakia, choroidal hemangioma, presence of rhegmatogenous retinal detachment, prior intraocular surgery, ocular trauma), and exposure to certain medications (anticoagulants, antiplatelet, and cardiovascular medications).2
Surgeon-related. Factors that have been noted to increase the intraoperative risk of suprachoroidal hemorrhage include low-volume cataract surgeons, novice surgeons, and surgeon case mix (ie, surgeons who regularly deal with more complex cases are more likely to face intraoperative complications).2,7,10
Intraoperative. These factors include prolonged or complicated surgery (commonly from vitreous loss), general and retrobulbar anesthesia, failure to administer ocular compression to reduce IOP before or after the retrobulbar delivery of anesthesia, intraoperative systemic hypertension, elective extracapsular cataract extraction (ECCE), and conversion from phacoemulsification to ECCE.
Postoperative. These factors include hypotony, valsalva maneuvers (eg, coughing, straining, postoperative emesis), and tissue plasminogen activator administration.
The prevention of suprachoroidal hemorrhage includes an assessment of modifiable risk factors (eg, keeping anticoagulant and antiplatelet medications at the lowest level possible before surgery, postponing nonurgent ocular surgery if the patient presents with cough); IOP control (eg, orbital compression when using peri- or retrobulbar anesthesia); and adequate management of comorbidities. In the postoperative period, hypotony should be avoided, and follow-up should be frequent for patients with the aforementioned risk factors.11-13
DIAGNOSIS
Clinical signs that suggest ASCH include an abrupt loss of the red reflex; hardening of the globe from an increase in IOP; a shallow anterior chamber; prolapse of intraocular contents, including the iris and vitreous, with wrinkling and bulging of the posterior capsule; and, in severe cases, corneal edema. Ling et al reported that ASCH was more likely to be limited with phacoemulsification (63.2%) compared to ECCE (11.1%) and phaco conversion (23.1%).7 ASCH also occurred more frequently after nucleus removal.
In contrast, patients with DSCH typically present to the office during the week after surgery with a complaint of blurry vision, headache, nausea, and/or vomiting. Clinical examination shows decreased visual acuity that cannot be corrected and choroidal elevation.7,12
The diagnosis of suprachoroidal hemorrhage is usually clinical, but imaging is often required to provide support, monitor progression, select the most appropriate treatment strategy, and rule out other pathologies. The gold standard for diagnosis is B-scan ultrasound, which helps to identify the location and extent of a hemorrhage and the status of the retina and vitreous. B-scan ultrasound is considered essential for determining the appropriate timing of future surgical intervention, if selected.10,11
OCT and MRI can also be implemented. Similar to ultrasound, OCT allows localization and measurement of the extent of suprachoroidal hemorrhage. MRI may help to differentiate between various soft tissue, serous, and hemorrhagic pathologies.11
MANAGEMENT
Even with prompt identification and intervention, the prognosis after suprachoroidal hemorrhage is poor. Most patients do not regain their prehemorrhage visual acuity or recover 20/200 VA or better. Treatment differs for ASCH and DSCH.
ASCH. The management of ASCH requires expeditious wound closure to tamponade the bleeding choroidal vessels and prevent intraocular contents from prolapsing. Deepening the anterior chamber by injecting an OVD or air can help to stabilize IOP and prevent the extension of a hemorrhage. Posterior draining sclerotomy at the time of suprachoroidal hemorrhage development is a controversial practice. 7,11,13,14
DSCH. The initial treatment of DSCH is usually conservative. Systemic steroids together with the topical application of intensive steroids have been shown to improve visual outcomes by controlling inflammation and promoting clot liquefaction. IOP control with the use of ocular hypotensive medication, pain control with the use of cycloplegics, and daily follow-up with routine BCVA and IOP measurements are needed. B-scan ultrasound is useful for monitoring clinical evolution.1,8,10
Severe cases of DSCH are often treated surgically. The most common procedure is sclerotomy to drain blood from the suprachoroidal space. Sclerotomy combined with vitrectomy is another option in some cases, as is internal tamponade using either gas or perfluorocarbon to optimize drainage. It is usually recommended to proceed with drainage 1 to 2 weeks after diagnosis to allow blood liquefaction.11,13,14
CONCLUSION
There is no standard management protocol for suprachoroidal hemorrhage, and prevention is therefore key. Adjusting modifiable risk factors in the pre-, intra-, and postoperative settings is essential to obtaining the best surgical outcomes. Patients with risk factors for suprachoroidal hemorrhage who recently underwent cataract surgery require close monitoring for signs and symptoms of DSCH. Clinicians should maintain a high index of suspicion so that treatment can be initiated promptly.
1. Foo R, Tsai A, Lim L. Management of suprachoroidal hemorrhage. EyeNet Magazine. May 2018. Accessed March 4, 2021. https://www.aao.org/eyenet/article/management-of-suprachoroidal-hemorrhage
2. Stein JD. Serious adverse events after cataract surgery. Curr Opin Ophthalmol. 2012;23(3):219-225.
3. Anand-Apte B, Hollyfield JG. Developmental anatomy of the retinal and choroidal vasculature. In: Dart DA, ed. Encyclopedia of the Eye. Academic Press; 2010:9-15.
4. Schrieber C, Liu Y. Choroidal effusions after glaucoma surgery. Curr Opin Ophthalmol. 2015;26(2):134-142.
5. Reibaldi M, Longo A, Romano MR, et al. Delayed suprachoroidal hemorrhage after pars plana vitrectomy: five-year results of a retrospective multicenter cohort study. Am J Ophthalmol. 2015;160(6):1235-1242.e1.
6. Hayreh SS. Posterior ciliary artery circulation in health and disease: the Weisenfeld lecture. Invest Ophthalmol Vis Sci. 2004;45(3):749-757; 748.
7. Learned D, Eliott D. Management of delayed suprachoroidal hemorrhage after glaucoma surgery. Semin Ophthalmol. 2018;33(1):59-63.
8. Chu TG, Green RL. Suprachoroidal hemorrhage. Surv Ophthalmol. 1999;43(6):471-486.
9. Bandivadekar P, Gupta S, Sharma N. Intraoperative suprachoroidal hemorrhage after penetrating keratoplasty: case series and review of literature. Eye Contact Lens. 2016;42(3):206-210.
10. Mantopoulos D, Hariprasad SM, Fine HF. Suprachoroidal hemorrhage: risk factors and diagnostic and treatment options. Ophthalmic Surg Lasers Imaging Retina. 2019;50(11):670-674.
11. Song W, Zhang Y, Chen H, Du C. Delayed suprachoroidal hemorrhage after cataract surgery: a case report and brief review of literature. Medicine (Baltimore). 2018;97(2):e8697.
12. Wang LC, Yang CM, Yang CH, et al. Clinical characteristics and visual outcome of non-traumatic suprachoroidal haemorrhage in Taiwan. Acta Ophthalmol. 2008;86(8):908-912.
13. Ling R, Cole M, James C, Kamalarajah S, Foot B, Shaw S. Suprachoroidal haemorrhage complicating cataract surgery in the UK: epidemiology, clinical features, management, and outcomes. Br J Ophthalmol. 2004;88(4):478-480.
14. Ghorayeb G, Khan A, Godley BF. Delayed suprachoroidal hemorrhage after cataract surgery. Retin Cases Brief Rep. 2012;6(4):390-392.




