Greater Confidence With Trifocal Toric IOLs

Robert Edward Ang, MD
Manila, Philippines
Trifocal IOLs have become my primary choice for presbyopia correction during cataract surgery. The rate of spectacle independence is very high, and surprisingly, complaints of glare and halos and problems with contrast sensitivity are minimal. The key to achieving a successful outcome and high patient satisfaction is minimizing residual refractive error after surgery.
The latest generation of IOL formulas has increased the accuracy with which spherical refractive error is corrected. Correcting astigmatism is trickier because of patients’ varying degrees of preexisting corneal astigmatism and because the effects of the incision and corneal wound healing can be unpredictable.
Five years ago, I considered a toric lens if the eye had at least 1.00 D of corneal cylinder. Two years ago, I began using the Barrett Toric Calculator for eyes with at least 0.50 D of corneal cylinder to see if it suggests a toric IOL—a recommendation I follow most of the time. A study published in 2019 convinced me that 0.75 D of astigmatism or more affects patients’ vision with trifocal IOLs.1 I therefore find myself implanting more trifocal toric IOLs than trifocal nontoric IOLs (at a rate of approximately 70% vs 30%, respectively).
DESIGN PREFERENCES
Of the available lenses, my usual preference is the FineVision Toric IOL (model POD FT, PhysIOL). The main reason is the ease of dialing the lens to the intended axis inside the capsular bag (Figure 1). Because of its double C-loop haptic design, this lens fits snugly into the equator and does not spin uncontrollably inside the capsular bag, even in an eye with a long axial length or large capsular bag. After removing the OVD from behind the lens, I can gently and easily rotate the IOL clockwise or counterclockwise.
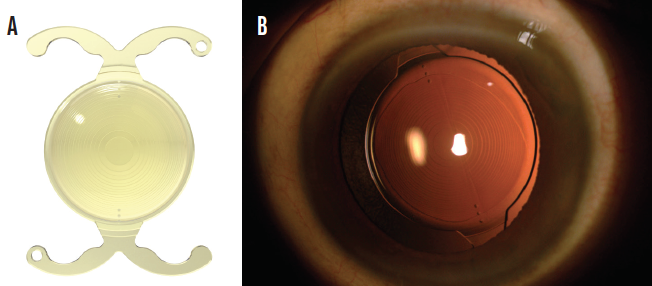
Figure 1. The design of the FineVision Trifocal Toric IOL (A). This lens in situ (B).
Courtesy of Robert Edward Ang, MD
I can get irritated with C-loop toric IOLs because, if I mistakenly rotate the lens beyond the desired axis, I have to rotate it around 180º to reposition it. Sometimes, I have to reinflate the capsular bag with an OVD to reposition the lens without stretching the bag or the zonules.
STUDY RESULTS
My colleagues and I are currently performing a prospective study to evaluate outcomes with the FineVision Toric IOL in eyes that have at least 1.00 D of corneal astigmatism. Of the 118 eyes enrolled, 98 have reached the 6-month follow-up visit. Mean preoperative corneal cylinder was 1.36 D, and the highest amount of cylinder was 4.56 D. Six months after surgery, mean sphere was 0.17 ±0.39 D, mean cylinder was -0.51 ±0.41 D, and the mean manifest refractive spherical equivalent was -0.08 ±0.31 D. Mean monocular uncorrected distance visual acuity was 0.06 ±0.11 logMAR, mean uncorrected intermediate visual acuity was 0.08 ±0.13 logMAR, and mean uncorrected near visual acuity was 0.10 to 0.12 logMAR. An independent reading center graded mean IOL rotation at 1.16 ±1.17º from hour 1 to day 1 and 1.66 ±2.69º from hour 1 to month 6.
Ease-of-use, my experience, and our study results thus far give me confidence in using this trifocal toric IOL more often in clinical practice.
1. Ang RE. Comparison of tolerance to induced astigmatism in pseudophakic eyes implanted with small aperture, trifocal, or monofocal intraocular lenses. Clin Ophthalmol. 2019;13:905-911.
A Surgeon-Changing Experience: The Development of the Presby-EDOF Formula

Detlev R.H. Breyer, MD
Düsseldorf, Germany
In 2019, I was invited by the International Society of Presbyopia to lecture on decision-making in presbyopia correction. When preparing my talk, I thought about Albert Einstein, who famously said that one should isolate the problem before finding a solution, and about Warren Buffet, who has urged presenters to imagine an auditorium filled not with peers but with lawyers, judges, and journalists. I crafted my talk with these principles in mind and focused on presenting research and scientific evidence.
ISOLATING THE PROBLEMs
Problem No. 1. Considering the complication rate, depth of treatment, potential for reversibility, and retreatment options for presbyopia-correcting procedures, I believe the only logical order is to consider laser vision correction (LVC) first, then phakic IOL implantation, and finally refractive lens exchange. LVC is generally safer than wearing contact lenses,1 whereas intraocular surgery carries a greater risk for complications and has worse retreatment options.
Problem No. 2. Photopic phenomena are the most common causes of patient dissatisfaction and IOL explantation. I have found that the best strategy by which to overcome this optical photopic dilemma in the correction of presbyopia is to split the near addition between both eyes and extend depth of focus by adding negative or positive spherical aberration. This can be achieved with PresbyMax (Schwind eye-tech-solutions) and Presbyond LVC (Carl Zeiss Meditec) procedures, which convert monovision into more tolerable blended vision.
PROOF OF (A NEW) CONCEPT
Blended vision is my preferred presbyopia-correcting strategy for patients. When I began developing presbyopia in my eyes, however, the question became whether I was ready to put my money where my mouth was.
In 2020, I decided to undergo Presbyond. I asked Dan Z. Reinstein, MD, MA(Cantab), FRCSC, DABO, FRCOphth, FEBO, to perform the procedure, but the start of the COVID-19 pandemic forced us to postpone surgery. This delay gave me time to consider other options.
Around that time, I began using the Amaris laser (Schwind eye-tech-solutions). I modified the original PresbyMax formula to a more negative near target refraction (ie, from -0.89 to -1.50 D) and increased the induced negative spherical aberration (ie, from 1.25 to 1.75 D). I found that these modifications helped my patients to achieve better reading vision. As a result, I decided that the best procedure for me, at 53 years of age, was Smart Surface transepithelial PRK with this new Presby-EDOF Formula (Figure 2).
Figure 2. Dr. Breyer undergoes presbyopia correction with the Amaris laser.
RESULTS
Three days after my surgery, I was carrying my usual surgical case load (20–30 cases per day) and seeing 30 patients in my private practice. My near vision was not perfect, but it was good enough to allow me to perform my daily tasks without a problem. When looking at a distant target, the vision in my near eye was foggy. Nevertheless, I was enthusiastic about my freedom from glasses and my improved quality of life.
At the time of this writing, 9 months after surgery, I do not use glasses—although I admit that reading small print is possible only in very good lighting conditions. Luckily, I rarely read small print.
Here is a fun fact: Covering my far-dominant eye, I experience 6/10 photopic phenomena, but as soon as I uncover that eye, they vanish. Neural adaptation is impressive.
If I had to do it all over again, I would surely elect the same procedure. Interestingly, since undergoing transepithelial PRK, I find myself recommending the procedure to more of my patients. I have also recommended it to friends.
NEW IN 2021
This year, I find myself using a similar strategy with IOLs. I have been implanting the diffractive AT LARA IOL (Carl Zeiss Meditec) in the far-dominant eye and the trifocal AT LISA IOL (Carl Zeiss Meditec) in the near-dominant eye. I call this strategy Trifo + Vision (Figure 3) because it also provides the best binocular defocus capacity and homogenous vision of any trifocal IOL I have measured.2
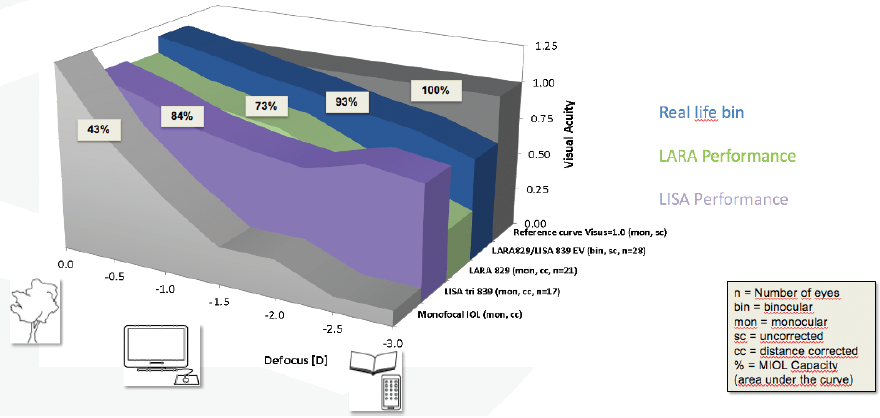
Figure 3. The Trifo + Vision strategy used by Dr. Breyer.
Figures 2 and 3 courtesy of Detlev R.H. Breyer, MD
1. Masters J, Kocak M, Waite A. Risk for microbial keratitis: comparative metaanalysis of contact lens wearers and post-laser in situ keratomileusis patients. J Cataract Refract Surg. 2017;43:67-73.
2. Tarib I, Diakonis VF, Breyer D, Höhn F, Hahn U, Kretz FTA. Outcomes of combining a trifocal and a low-addition bifocal intraocular lens in patients seeking spectacle independence at all distances. J Cataract Refract Surg. 2019;45(5):620-629.
A Progressive-Thickness ICRS for Keratoconus Treatment

Aline S. Moriyama, MD
São Paulo, Brazil
Visual disturbances in patients with keratoconus often can be managed with spectacles and rigid contact lenses.1 Visual rehabilitation, however, in patients with unsatisfactory BCVA who are intolerant of contact lenses can be challenging.2 I am excited this year to enhance surgical outcomes with new intrastromal corneal ring segments (ICRSs) with a thickness that becomes progressively greater.
BACKGROUND
ICRSs are PMMA implants that promote localized corneal flattening adjacent to where they are implanted.3 Greater flattening effect is seen in thicker implants with smaller apical diameters. ICRSs can help to regularize corneal shape, reduce the amount of astigmatism in the eye, and improve visual acuity.3,4 They can be removed if necessary, and surgery is relatively straightforward, especially when a femtosecond laser is used to create the implantation tunnel.5
In my experience, ICRSs are appropriate for patients with keratoconus who meet the following criteria:
- Unsatisfactory BCVA and contact lens intolerance;
- No visually significant corneal opacity;
- A desire to avoid or postpone corneal transplantation or live in regions where access to corneal tissue for transplantation is poor; and
- Realistic expectations and an understanding of ICRS surgery, its limitations, and the predictability of its outcomes.
In Brazil, where I practice, the most widely used ICRSs are the Keraring (Mediphacos) and the Ferrara Ring (Ferrara Ophthalmics). Traditionally, these devices have triangular arcs with angles between 90º and 330º and a thickness between 150 and 350 µm. ICRSs with a smaller angle are generally used to treat astigmatism by either implanting a single segment or a combination of two segments. ICRSs with 320º or greater arc usually generate maximal central applanation with minimal astigmatism alterations. The best indications for an ICRS of this arc angle is central keratoconus with low astigmatism.
Over the past few years, ICRS models have come to market with variable thickness along the length of the arc.6,7 Most patients with keratoconus have asymmetric astigmatism with an inferior corneal steepening. The amount of corneal flattening is related to ICRS thickness; progressive-thickness ICRSs are therefore preferred to treat astigmatism with significant asymmetry.
I have been using 160º Keraring arcs for a few years (Figure 4A), and my results thus far are encouraging. The thickness of the arcs progresses either from 150 to 250 µm or from 200 to 300 µm. I use a single progressive-thickness Keraring for patients with keratoconus and irregular and asymmetric astigmatism. I combine two of these ICRSs for patients with keratoconus and regular asymmetric astigmatism.
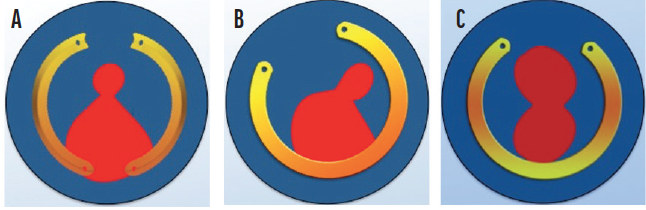
Figure 4. Keraring variable-thickness ICRS models have a 100-µm difference in thickness along the arc. In this diagram, the thinnest part of the ICRS is represented in yellow and the thicker part in orange. Combination of ICRSs with a 160º arc whose thickness varies along the length of the arc (A). An ICRS with a 330º arc has thinner tips and a thicker central area (B). An ICRS with a 320º arc is thinner centrally and thicker laterally (C).
Courtesy of Mediphacos
Recently, 320º and 330º models of the Keraring with variable thickness along the arc length were released (Figure 4B and C).8 These can be used to treat corneal astigmatism when the central keratometry reading is high. Availability of a wide range of Keraring models allows more customized treatment for patients with keratoconus (Figure 5), and I look forward to using the devices in 2021 and beyond.
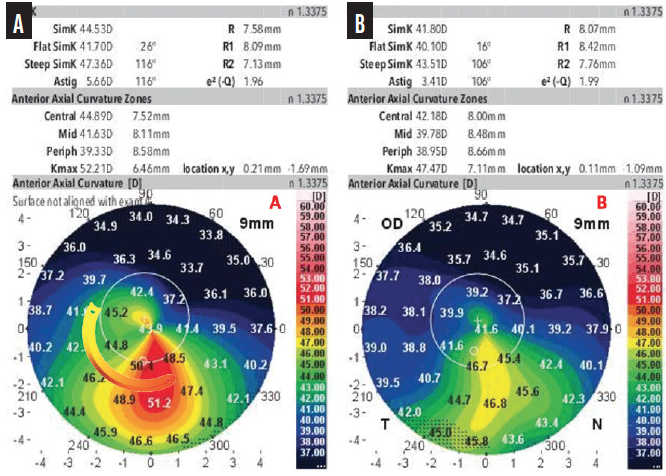
Figure 5. A preoperative anterior axial curvature map shows irregularity and asymmetry in a patient who is intolerant of contact lenses (A). The thickness of the selected ICRS with a 160º arc increases from 150 to 250 µm. The postoperative examination showed a reduction in astigmatism and keratometry (B). BCVA improved from 20/80 with a manifest refraction of -3.50 -4.00 x 25º to 20/30 with a manifest refraction of -0.75 -2.50 x 10º.
Courtesy of Aline S. Moriyama, MD
1. Mohammadpour M, Heidari Z, Hashemi H. Updates on management for keratoconus. J Curr Ophthalmol. 2018;30(2):110-124.
2. Parker JS, van Dijk K, Melles GRJ. Treatment options for advanced keratoconus: a review. Surv Ophthalmol. 2015;60(5):459-480.
3. Vega-Estrada A, Alio JL. The use of intracorneal ring segments in keratoconus. Eye Vis. 2016;3(1):8.
4. Park SE, Tseng M, Lee JK. Effectiveness of intracorneal ring segments for keratoconus. Curr Opin Ophthalmol. 2019;30(4):220-228.
5. Park J, Gritz DC. Evolution in the use of intrastromal corneal ring segments for corneal ectasia. Curr Opin Ophthalmol. 2013;24(4):296-301.
6. Baptista PM, Marques JH, Neves MM, Gomes M, Oliveira L. Asymmetric thickness intracorneal ring segments for keratoconus. Clin Ophthalmol. 2020;14:4415-4421.
7. Prisant O, Pottier E, Guedj T, Hoang Xuan T. Clinical outcomes of an asymmetric model of intrastromal corneal ring segments for the correction of keratoconus. Cornea. 2020;39(2):155-160.
8. Mediphacos. Keraring Catalog. Accessed March 12, 2021. http://mediphacos.com/wp-content/uploads/2020/10/Brochure-Keraring-3009-SM.pdf
Looking Forward to Using a New Monofocal IOL

Roberto Pineda, MD
Massachusetts, United States
One of the great things about ophthalmology is that technology changes rapidly to facilitate the diagnosis and management of different disorders. I look forward to incorporating a few new technologies into my practice every year. This year, I am especially excited about the Tecnis Eyhance (model ICB00, Johnson & Johnson Vision), which was approved by the FDA in February.
This IOL is built on the Tecnis platform, is available in nontoric and toric models (Tecnis Eyhance and Tecnis Eyhance Toric II), and comes with the Tecnis Simplicity Delivery System. The lens is available in a wide range of powers (+5.00 to +34.00 D). The toric version can correct up to 4.00 D of astigmatism at the corneal plane, and its haptics are squared and frosted to minimize IOL rotation.
EXPLORING THE TECHNOLOGY
Benefits. The recent 2020 Market Scope Global IOL report stated that 84% of ophthalmic surgeons implant monofocal IOLs during cataract surgery. For me, the main benefits of the Tecnis Eyhance IOL are its central increased zone of negative asphericity (described as a higher-order polynomial aspheric anterior surface), its delivery of image contrast in low-light settings, and its injector to increase the safety and ease of delivery while protecting against contamination.
Experience. This IOL was introduced 1 year ago in Europe and Canada and in Latin America this past summer. International colleagues who have used this IOL have told me that their experiences have been positive, and some have made it their standard monofocal IOL. They are reporting enhanced intermediate vision with targets of -0.25 D in the distance eye and -0.50 to -0.75 D in the nondominant eye to achieve J3 or J2 near vision in many patients.
Indications. Many multifocal and some extended depth of focus (EDOF) IOLs feature diffractive optics. This design limits their use in patients with a history of refractive surgery, glaucoma, and age-related macular degeneration owing to the risk of unwanted visual side effects from higher-order aberrations and visual underperformance, particularly in low-contrast environments. The Tecnis Eyhance may offer an alternative to a traditional monofocal IOL for some of these patients.
CONClUSION
I look forward to offering the Tecnis Eyhance IOL. My first case is already scheduled. I believe that many of my patients will benefit from this technology, and I think that it fits well with my objective to underpromise and overdeliver on visual results to my cataract patients.
Excited for Technologies That Address Refinements in Cataract Surgery

Patrick Versace, MD
Sydney, Australia
More than 20 million cataract procedures are performed globally each year. With that annual volume and, in this, the 21st year of the 21st century, we have cataract surgery perfected—right?
Not really. But, we surgeons have had an opportunity to refine each element of the procedure. The good news is that, today, we can pay more attention to detail now that the safety and visual results achieved with cataract surgery are typically excellent.
TWO TECHNOLOGIES
The new interventions I plan to incorporate this year aim to refine aspects of cataract surgery in ways that were impossible until now.
No. 1: Laser capsulotomy. The CapsuLaser (Excel-Lens) creates a capsulotomy with precise size and positioning (Figure 6). This device allows me to create a capsulotomy that is 1.5 times stronger than a manual capsulorhexis and three times stronger than a femtosecond laser capsulotomy1 but at a fraction of the cost to patients. The compact unit is mounted on an operating microscope. The CapsuLaser uses a 590-nm laser to create the capsulotomy (scan the QR code now to watch a related video). Trypan blue dye (at a concentration of 0.4%) is used to stain the anterior capsule and to selectively absorb the laser energy that converts type 4 collagen to amorphous collagen.

Figure 6. A CapsuLaser capsulotomy.
A strong, perfectly positioned capsulotomy of predictable size is important for the second refinement I am incorporating this year.
No. 2: Capsulotomy fixation of an IOL. The Femtis family of IOLs (Teleon Surgical) is designed to fixate to the anterior capsulotomy (Figure 7). These lenses are available in three optical designs: EDOF, toric, and EDOF toric. Alignment of the EDOF toric IOL with the visual axis is predictable in my experience (Figure 8) and achieves on-axis cylinder correction within 1.5º of the intended axis.2 Alignment with the visual axis removes one of the variables that is responsible for unexpected visual outcomes with EDOF and multifocal IOLs.
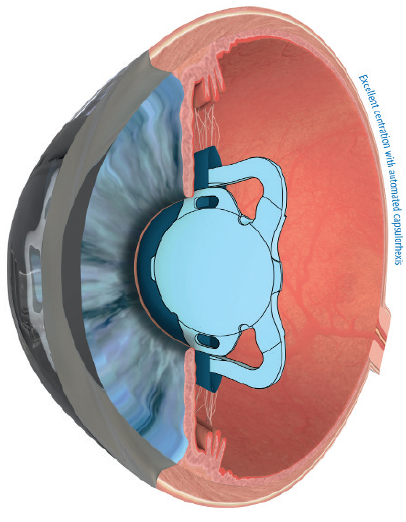
Figure 7. Schematic of the position of the Femtis IOL.
Courtesy of Teleon Surgical
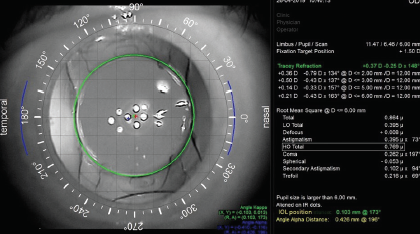
Figure 8. An iTrace (Tracey Technologies) image of the Femtis IOL aligned with the visual axis.
Figures 6 and 8 courtesy of Patrick Versace, MD
The fixation of the Femtis IOL to the capsulotomy provides a more predictable effective lens position in the plane of the anterior capsule. This improves refractive predictability, significantly reduces lens tilt compared to in-the-bag fixation, and theoretically reduces the induction of coma. Overall, fixating the IOL to the capsulotomy gives me more control over the mechanical elements of lens implantation that can affect visual performance (scan the QR code now to watch a related video).
The Femtis EDOF IOL has the same asymmetric refractive segmental optical design as the Lentis Comfort, Lentis M plus, and Lentis M plus X (all from Teleon Surgical), which provides my patients with good functional vision. In my practice, in Australia, 60% of patients achieve spectacle independence and report minimal unwanted visual phenomena.3
CONCLUSION
The combination of the CapsuLaser with a Femtis IOL allows me to offer more patients functional vision with an extended depth of focus. It also tightens the range of outcomes I achieve by eliminating the outliers that might have occurred because of IOL decentration, IOL tilt, and dysphotopsias.
1. Daya S, Chee SP, Ti SE, Packard R, Mordaunt DH. Comparison of anterior capsulotomy techniques: continuous curvilinear capsulorhexis, femtosecond laser-assisted capsulotomy and selective laser capsulotomy. Br J Ophthalmol. 2020;104(3):437-442.
2. Auffarth GU, Friedmann E, Breyer D, et al. Stability and visual outcomes of the capsulotomy-fixated FEMTIS-IOL after automated femtosecond laser-assisted anterior capsulotomy. Am J Ophthalmol. 2021;225:27-37.
3. Darian-Smith E, Versace P. Visual performance and positional stability of a capsulorhexis-fixated extended depth-of-focus intraocular lens. J Cataract Refract Surg. 2020;46(2):179-187.
Enhancing Patient Care With Two New Offerings

Dagny Zhu, MD
California, United States
When evaluating new technologies, I tend to think of them in relation to how they may enhance patient care. In 2021, two technologies that I believe will have a positive effect on patient care are the AcrySof IQ Vivity Extended Vision IOL (Alcon) and the Systane iLux MGD Thermal Pulsation System (Alcon). A third technology, the Evo Visian ICL (STAAR Surgical), should it be approved by the FDA this year, would also be a welcome addition to my offerings.
THREE TECHNOLOGIES
AcrySof IQ Vivity Extended Vision IOL. My experience with this IOL has been very positive. I started implanting this lens in the winter, and at the time of this writing, I have placed about 75 (Figure 9). It is the first and only nondiffractive EDOF IOL, and patients have been very pleased with their outcomes. The nondiffractive design of the Vivity IOL provides them with a continuous extended range of vision and a low incidence of visual disturbances.1
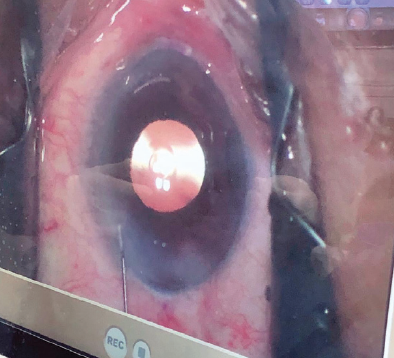
Figure 9. The AcrySof IQ Vivity Extended Vision IOL in situ.
Courtesy of Dagny Zhu, MD
The XWave nondiffractive technology of the Vivity creates an extended focal range by stretching and shifting the wavefront rather than splitting the wavefront into multiple focal points like diffractive multifocal IOLs.2 I find this technology is a great adjunct to the presbyopia-correcting IOLs I offer to patients in my practice. I gravitate toward the AcrySof IQ PanOptix Trifocal IOL (Alcon) in many patients. Not everyone, however, is a good candidate for it, including those with a poor ocular surface or severe pathology and those who may not tolerate the dysphotopsias commonly associated with diffractive technology.
The Vivity IOL is great for patients who want a fuller range of vision than they would get with a traditional monofocal IOL, and patients are impressed with the intermediate vision and functional near vision (around J3) they can achieve with this lens. It also has a reduced risk of halos and glare and fewer contrast sensitivity issues commonly associated with diffractive multifocal IOL technologies. In the FDA studies, the visual disturbance profile of the Vivity was similar to the monofocal AcrySof IOL (Alcon). I am happy to report that this also has been my clinical experience. The only drawback, of course, is that patients must be comfortable with using readers occasionally for smaller print and seeing things up close. I have found, however, that I can sometimes achieve better near vision by targeting slight myopia (-0.50 D) in the nondominant eye for a micro-monovision or blended approach. In my experience, when patients are counseled properly, they’re happy with the range of vision that they are able to obtain with the Vivity IOL.
Systane iLux MGD Thermal Pulsation System. I recently acquired the iLux. With an 8- to 12-minute treatment performed bilaterally, I can help optimize the ocular surface for some of my patients preoperatively so that they can experience enhanced results postoperatively. Four weeks after this treatment, meibomian gland function can increase by as much as 300%.3
Evo Visian ICL. I hope to be able to gain experience with the Evo Visian ICL later this year, depending on its approval status. I’m excited about this technology, which has a hole in the center of the optic, because it will eliminate the need to perform a peripheral iridotomy and theoretically decrease the risk of not only postoperative IOP issues but cataract formation as well.
1. Alcon announces European launch of Vivity, the only presbyopia-correcting intraocular lens with x-wave technology [news release]. Alcon. March 12, 2020. Accessed March 16, 2021. https://www.alcon.com/media-release/alcon-announces-european-launch-vivity-only-presbyopia-
correcting-intraocular-lens-x
2. Ligabue E, Ang RE, Dick HB, et al. In the pipeline. Cataract & Refractive Surgery Today. April 2020. Accessed March 16, 2021. https://crstoday.com/articles/2020-apr/in-the-pipeline/
3. Tauber J, Owen J, Bloomenstein M, Hovanesian J, Bullimore MA. Comparison of the iLUX and the LipiFlow for the treatment of meibomian gland dysfunction and symptoms: a randomized clinical trial. Clin Ophthalmol. 2020;14:405-418.




