Fluctuating Vision After SMILE
Farhad Hafezi, MD, PhD, FARVO

A 35-year-old woman presented to our clinic in October 2016 with a complaint of dryness in her right eye and poor, unstable vision in her left. She had undergone SMILE for the correction of -4.00 D of myopia in both eyes at another clinic 4 months before the consultation. The IOP in the patient’s left eye was 8 mm Hg, and the optical zone was small and decentered.
Some irregularity was observed in the interface of the left eye, but there were no remnants of the lenticule. Ocular aberrometry (Peramis, CSO Italia) showed a massive increase in the wavefront error (root mean square height value 1.42 µm at a 7-mm pupil) that predominantly consisted of spherical aberration and vertical coma. The cornea’s biological response to laser surgery can continue for months after refractive surgery, so we decided to wait until 6 months after the SMILE surgery before commencing treatment.
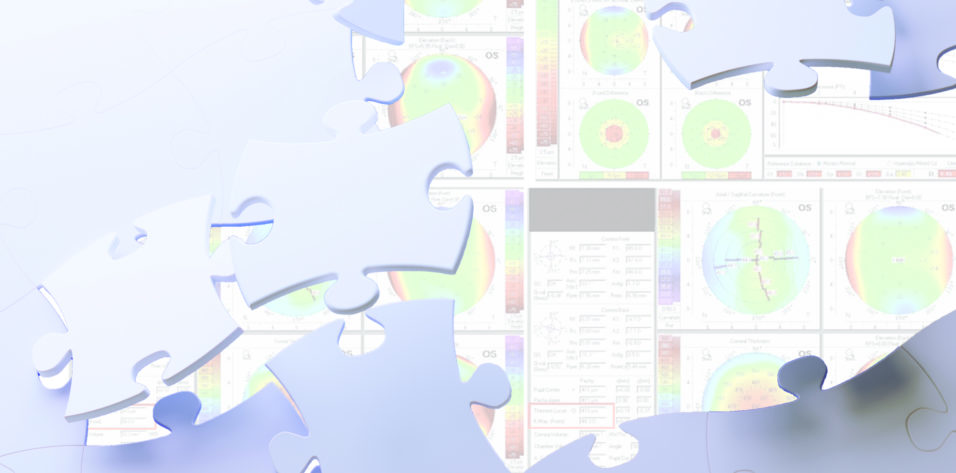
The patient returned in January 2017, and nothing had changed. We performed a comprehensive assessment of the cornea of the left eye (+0.25 -0.50 x 45º = 0.50) and planned a corneal wavefront-guided transepithelial PRK (trans-PRK) procedure with the Amaris 750S excimer laser (Schwind eye-tech-solutions). We recommended this option as opposed to other approaches such as a cap-to-flap conversion to a LASIK procedure and repeat SMILE partly because we wished to maintain the greatest corneal biomechanical integrity possible.1 Additionally, the patient had expressed a desire not to undergo another SMILE procedure. The trans-PRK procedure was performed 2 weeks later with a larger optical zone (Figure 1A).
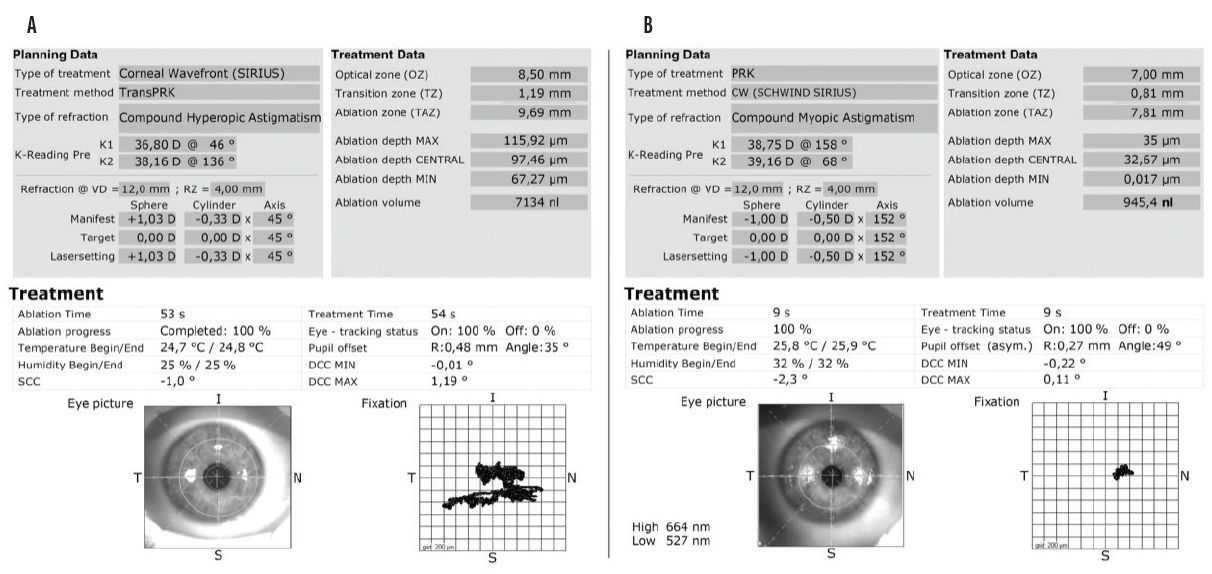
Figure 1. Planning and treatment data for the first (A) and second (B) trans-PRK procedures.
At the 6-month follow-up visit, the near visual acuity in the patient’s left eye had improved significantly (-0.75 -0.75 x 100º = 0.63). More importantly, the patient’s quality of vision had greatly improved. The IOP in this eye remained unchanged at 8 mm Hg.
We decided to perform a second corneal wavefront-guided trans-PRK procedure (Figure 1B) in October 2017. At the 6-month follow-up visit, the vision in the patient’s left eye had improved again (-0.50 -0.50 122º = 1.25), and the IOP was 10.5 mm Hg. The root mean square height value was now 0.33 at a 7-mm pupil (Figure 2). Distance vision in the left eye was not perfectly in focus. It had improved significantly, however, and the patient was extremely happy with the result.
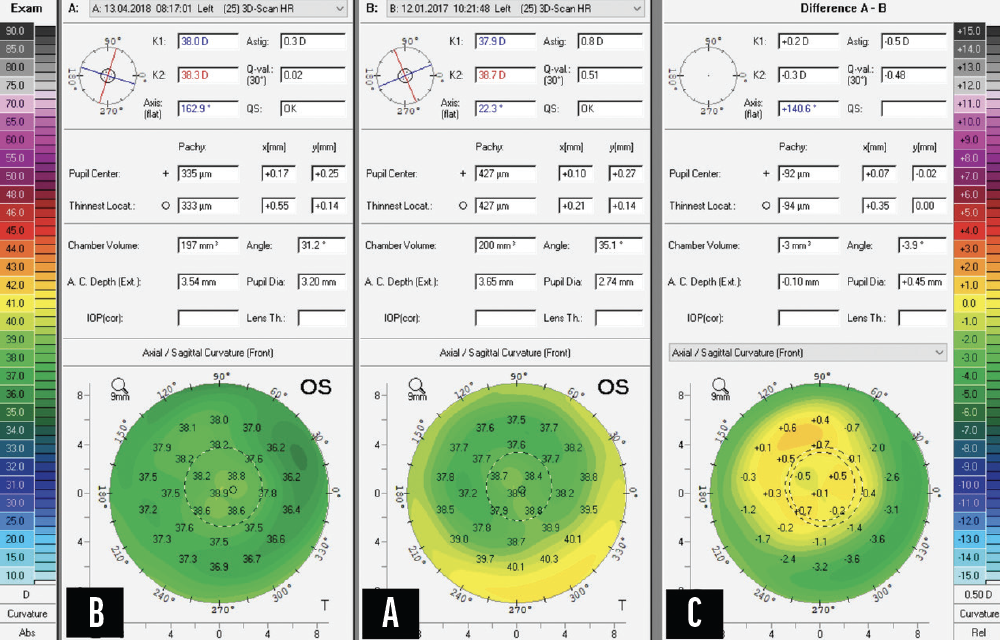
Figure 2. Scheimpflug imaging before (A) and after (B) the second trans-PRK procedure and the difference map (C).
Figures 1 and 2 courtesy of Farhad Hafezi, MD, PhD, FARVO
1. Kling S, Spiru B, Hafezi F, Sekundo W. Biomechanical weakening of different re-treatment options after small incision lenticule extraction (SMILE). J Refract Surg. 2017;33(3):193-198.
Pinhole Optics for PMD
Ashvin Agarwal, MS

Pellucid marginal degeneration (PMD) is a progressive degenerative corneal disorder characterized by thinning of the inferior cornea and a normal central cornea. My contribution to this article shares a case example in which a 50-year-old patient presented with a progressive deterioration of vision in the left eye. An examination revealed a cataract and 11.00 D of astigmatism due to PMD (Figure 3). The patient’s UCVA was 20/200.
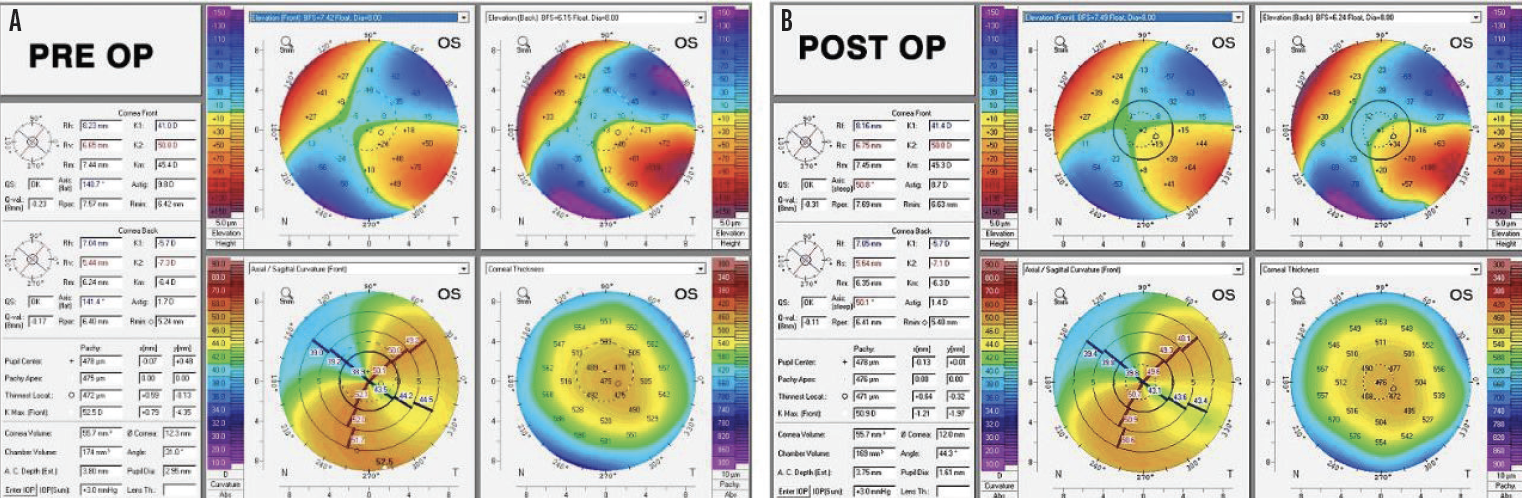
Figure 3. Preoperative (A) and postoperative (B) Scheimpflug images (Pentacam, Oculus Optikgeräte).
No specific treatment has been formulated for the management of corneal irregularity associated with PMD. I recommended combining the use of pinhole optics (at the pupillary plane) with cataract surgery. A toric IOL was avoided because of an increased risk of a refractive surprise due to irregular astigmatism and associated higher-order aberrations.
After phacoemulsification, a foldable one-piece hydrophobic IOL was implanted in the capsular bag. A pinhole pupilloplasty1,2 was initiated, and the pupil was centered around the Purkinje-1 reflex (light emanating from the surgical microscope; Figure 4).
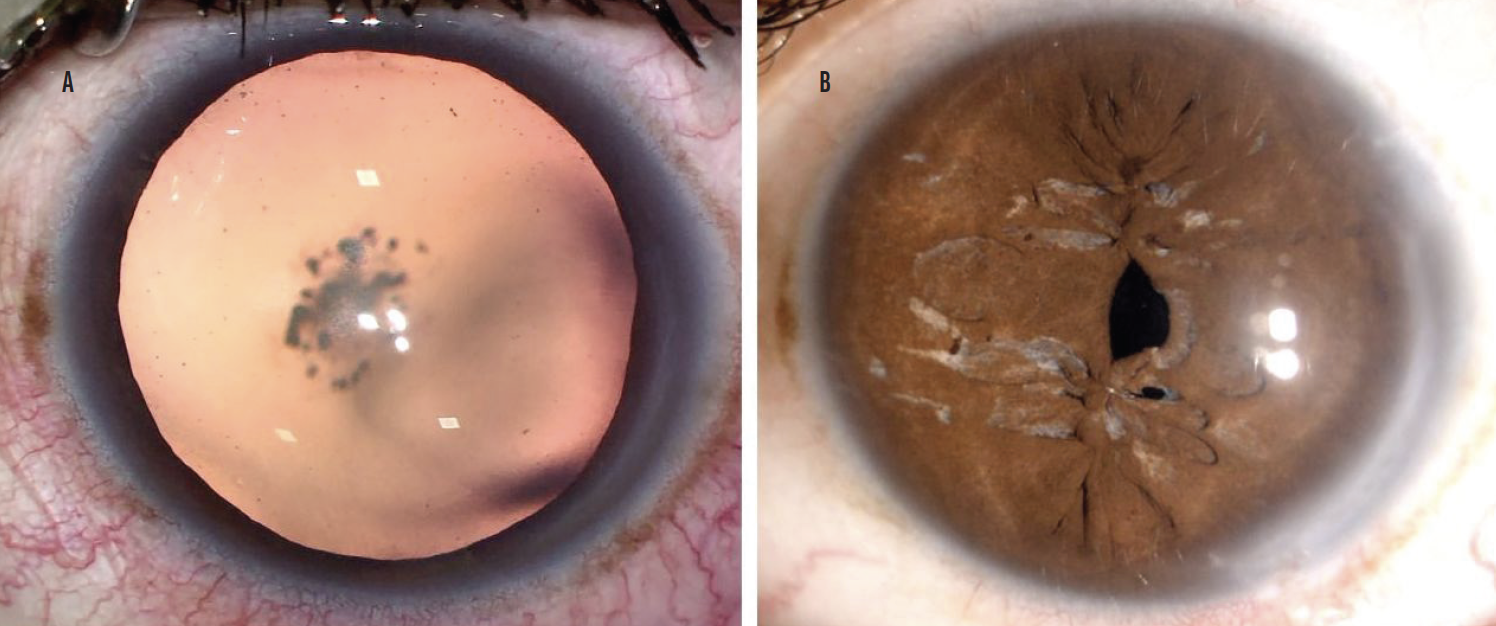
Figure 4. The eye before surgery (A) and following pinhole pupilloplasty (B).
Figures 3 and 4 courtesy of Ashvin Agarwal, MS
Recently, my colleagues and I started customizing the size of the pinhole by using a reticle that is integrated into the eyepiece of the microscope. A pinhole occluder with various sizes is used to identify what size pinhole maximizes the patient’s visual potential. An attempt is then made to achieve the same pinhole size intraoperatively.
I favor a single-pass four-throw pupilloplasty3 because I find the technique to be easy, simple, and fast and its results to be reproducible. One day after surgery, the patient’s UCVA was 20/40—a dramatic improvement.
1. Narang P, Agarwal A, Ashok Kumar D, Agarwal A. Pinhole pupilloplasty: small-aperture optics for higher-order corneal aberrations. J Cataract Refract Surg. 2019;45(5):539-543.
2. Narang P, Holladay J, Agarwal A, Jaganathasamy N, Kumar DA, Savanna S. Application of Purkinje images for pinhole pupilloplasty and relevance with chord length mu. J Cataract Refract Surg. 2019;45(6):745-751.
3. Narang P, Agarwal A. Single-pass four-throw technique for pupilloplasty. Eur J Ophthalmol. 2017;27(4):506-508.
Bilateral Keratoconus and Cataracts
Audrey R. Talley Rostov, MD

A 58-year-old woman with a long history of keratoconus and successful scleral contact lens use presented with a chief complaint of dry eye. She could no longer wear her contact lenses for more than a couple of hours and asked about alternatives. She had been wearing glasses for several months before she came to see me for a consultation. The patient had been told by her optometrist that she had early cataracts.
On initial examination, her BCVA with a contact lens was 20/40 OD (manifest refraction -6.75 +4.50 x 135º = 20/60) and 20/80 OS (manifest refraction -13.75 +7.75 x 047º = 20/100). Corneal tomography is shown in Figure 5.
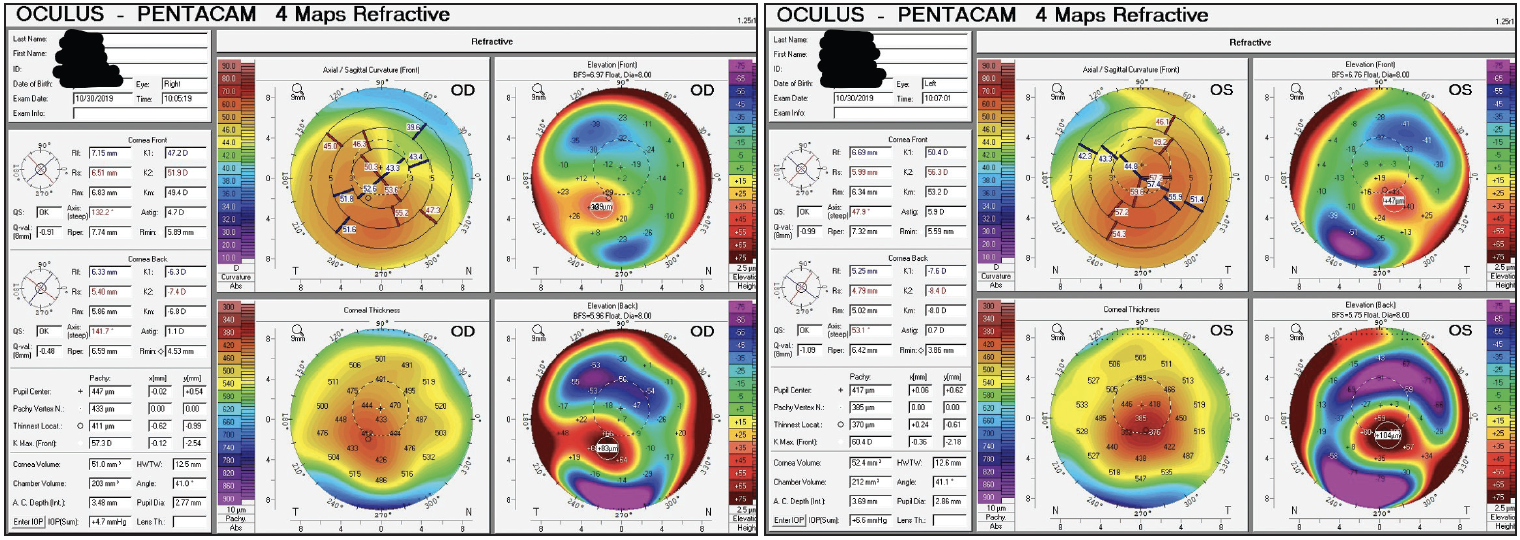
Figure 5. Tomography at the initial visit demonstrating keratoconus in both eyes.
The slit-lamp examination was significant for bilateral keratoconus. A Fleischer ring, mild superficial punctate keratitis, and a grade 1+ to 2+ nuclear sclerotic (NS) cataract were visible in the right eye. There was no apical scarring. In the left eye, the keratoconus was more severe with visible Vogt striae, mild apical scarring, grade 1+ superficial punctate keratitis, and a grade 1+ to 2+ NS cataract. The dilated funduscopic exam was normal in both eyes.
I had a long discussion with the patient to review her options for visual rehabilitation. I explained that CXL could help flatten the cornea in her right eye, which might improve the contact lens fit. Another option was cataract surgery in both eyes, but I noted that the visual potential of the left eye was limited by the apical scarring. I informed the patient that her best option for maximizing vision was to optimize the ocular surface and proceed with cataract surgery and implantation of a toric IOL in the right eye and a deep anterior lamellar keratoplasty (DALK) procedure in the left eye. Approximately 1 year after DALK and the removal of all sutures, cataract surgery would be performed on the left eye.
SURGICAL COURSE
The patient elected to have surgery on her left eye first because it was her worse-seeing eye and she felt that she still had functional vision in her right eye. I explained to the patient that the goal was to maximize her vision to the extent possible and that she would likely need to wear glasses or be refit with contact lenses to achieve improved—but not perfect—vision. The patient began administering lifitegrast ophthalmic solution 5% (Xiidra, Novartis) twice a day in each eye and was instructed to instill preservative-free tears at least three times per day and as needed thereafter.
The DALK procedure was uneventful. After 3 months, the sutures were gradually removed. The last sutures were removed 14 months postoperatively. At that time, the cataracts had progressed to grade 2+ NS in each eye, and scattered cortical changes were observed in the left eye. There were no other remarkable findings (Figure 6).
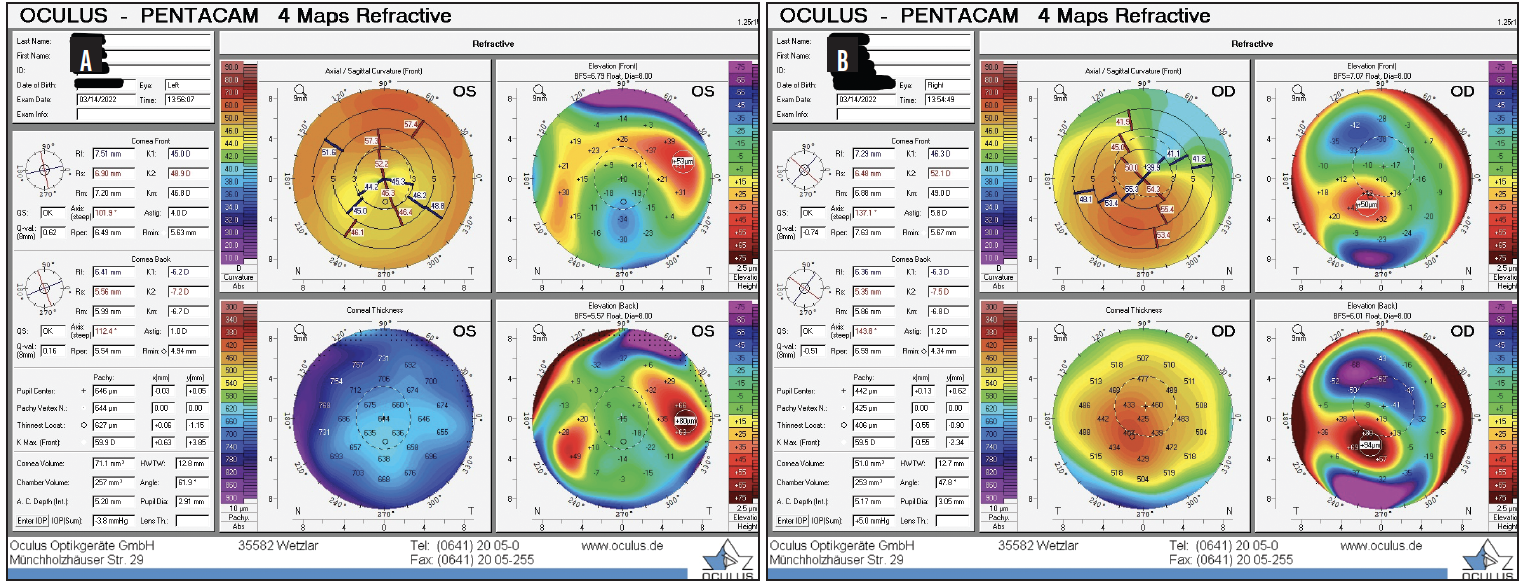
Figure 6. Postoperative tomography for the left (A) and right (B) eyes.
The result of the tomography for the right eye was unchanged since the initial visit, and the left eye now had regular astigmatism consistent with the DALK procedure on tomography. The patient’s BCVA was -4.25 +7.00 x 135º = 20/50 OD and -8.00 +5.25 x 110º = 20/60-2 OS. The results of biometry with the IOLMaster 700 (Carl Zeiss Meditec) are shown in Figure 7.
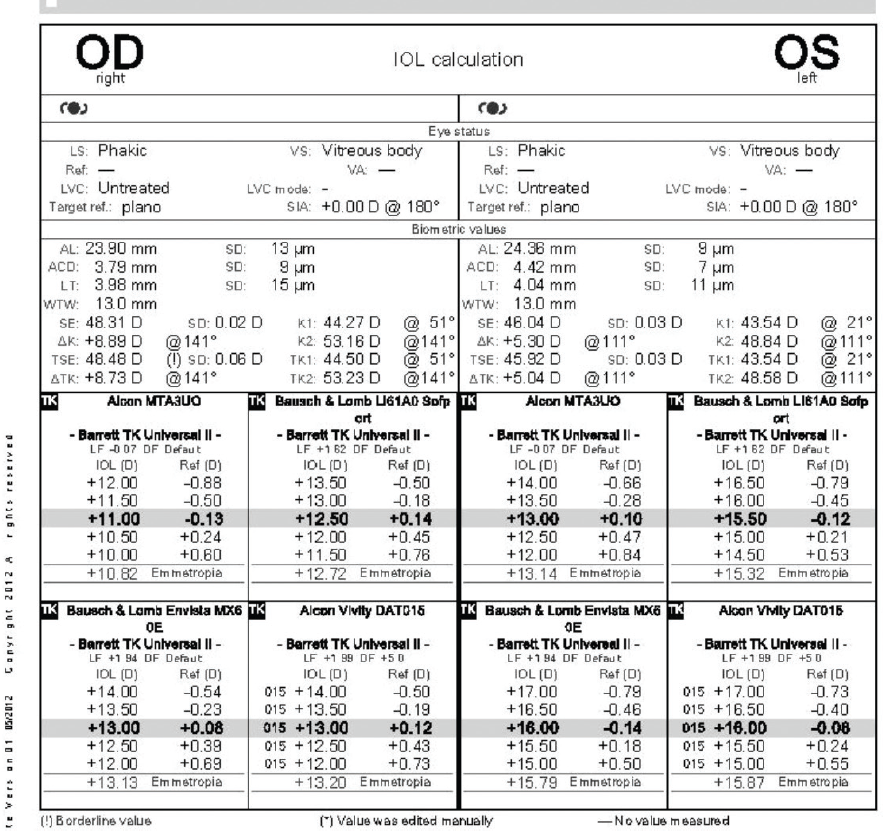
Figure 7. Biometry results.
Figures 5–7 courtesy of Audrey R. Talley Rostov, MD
Laser cataract surgery was performed on the left eye first. The Lensar Laser System (Lensar) was used to create a custom capsulotomy with alignment tabs to center the toric IOL. The use of the laser allowed phaco power to be reduced to minimize endothelial cell loss during surgery. A dispersive OVD was used to further minimize endothelial cell loss.
An enVista toric IOL (MX60T575, Bausch + Lomb) was implanted. This lens has a neutral aberration profile, which I find to be helpful for corneas with higher-order aberrations. The planned postoperative refraction was -0.75 D. Generally, I target slight myopia for patients with keratoconus and those undergoing penetrating keratoplasty and DALK because I find that all IOL formulas tend to produce hyperopic outcomes for these patients. Postoperatively, there were no complications, and the manifest refraction was -1.50 +1.00 x 160º = 20/20-2 OS.
Two weeks later, laser cataract surgery was performed on the patient’s right eye with a planned postoperative refraction of -0.75 D. An MX60T575 IOL was implanted at an axis of 135º to coincide with the patient’s corneal tomography, biometry, and Cassini (Cassini Technologies) readings. Her final UCVA and BCVA were 20/50 and +1.00 D = 20/25 OD and 20/40+1 and -1.50 +1.00 x 160º = 20/20-2 OS, respectively.
Postoperatively, both corneas were clear. The keratoconus was stable in the right eye, and the toric IOL was well centered on the 135º axis. The DALK graft in the left eye was clear, the toric IOL was well centered on the 110º axis, and the two corneal relaxing incisions in the DALK graft provided additional astigmatism management. The result in the right eye was more hyperopic than anticipated. The patient, however, was happy with her UCVA and BCVA. I offered to exchange the IOL in the right eye to correct the hyperopia, but she declined.
DISCUSSION
I have not found intraoperative aberrometry to be useful in situations such as this one. I create corneal relaxing incisions within the graft at an optical zone of 6.6 to 7.0 mm depending on the graft’s size. I do not open the incisions unless the toric IOL is unable to address all of the cylinder because doing so adds to the effect of the toric IOL in eyes with a history of penetrating keratoplasty and DALK. The incisions can be opened at the slit lamp postoperatively if more astigmatic correction is needed. Corneal relaxing incisions are never performed on patients with keratoconus because they could induce ectasia.
A complex cornea can present challenges, especially during cataract surgery. Combining toric IOLs with corneal relaxing incisions and/or PRK can optimize vision for these patients. It is important to set realistic goals for patients preoperatively.