Many options are available for the treatment of meibomian gland dysfunction (MGD), but most insurance plans cover only pharmaceuticals. Low-level light therapy (LLLT) is a low-cost entry point to MGD management. It is effective both as a standalone procedure with the Equinox (Espansione Group) and in combination with intense pulsed light (IPL) therapy with the Epi-C Plus (Espansione Group).1
MECHANISM OF ACTION
LLLT is a form of photobiomodulation (Figure 1). Athermal, atraumatic cellular activation is achieved through the application of light-emitting diodes of specific wavelengths. Photon interference characteristic of light-emitting diode use increases photo-intensity and penetration below the skin. Photoactivation results in the repair of damaged or compromised cells and an improvement in the functioning of healthy cells.2 Potential contributing effects include photoactivation in combination with warming of the meibum and minimization of Demodex mite infestation through increased phagocytic activity.3
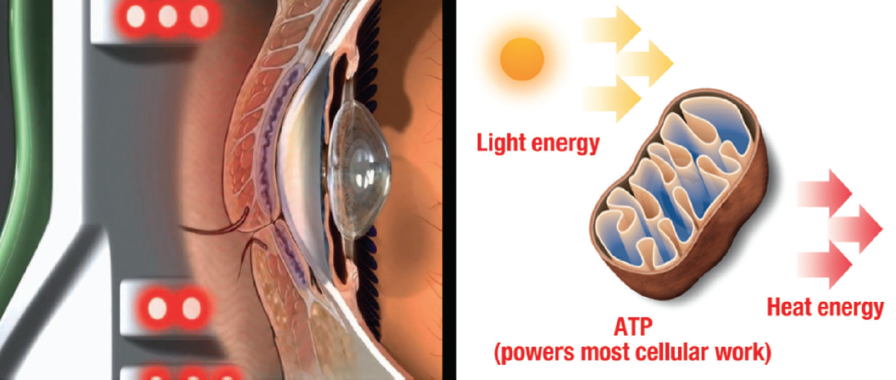
Figure 1. LLLT photobiomodulation mechanism of action. Abbreviation: ATP, adenosine triphosphate
Courtesy of Espansione Group
RESULTS TO DATE
Prospective trial of combined IPL and LLLT for the treatment of MGD. This study involved the treatment of 460 eyes of 230 patients for whom prior treatment with topical and systemic medications had failed.1
All of the patients underwent a complete ocular examination. The primary focus in this patient population was a subjective evaluation of combined IPL and LLLT using the Ocular Surface Disease Index (OSDI) and an objective evaluation of the intervention by means of meibomian gland expression (MGE) and tear breakup time (TBUT) performed before treatment and 1 to 3 months after treatment. Patients were observed for up to 1 year.
The average OSDI score decreased from 44.4 before treatment to 25.4 after treatment. MGE was rated on a 4-point scale; a score of 4 indicated an inability to express meibum, and a score of 0 indicated normalcy. The average MGE score decreased from 3.63 before treatment to 2.36 after treatment. The average TBUT increased from 3.78 seconds before treatment to 7.56 seconds after treatment.
No adverse events were reported. All patients noted an improvement in symptoms after treatment.
These results suggest that combined IPL and LLLT is beneficial as a primary or a secondary intervention for MGD in patients for whom topical and/or systemic therapy has failed.
Retrospective chart review. My colleagues and I evaluated the effects of LLLT on clinical measures of dry eye disease (DED) related to MGD in 50 eyes of 25 patients who had been unresponsive to prior treatment with pharmaceuticals and/or devices.4 Patients received three LLLT treatments over the course of 1 week with adjunctive administration of a topical fixed combination of a steroid and an antibiotic. Posttreatment diagnostic measurements were obtained 3 to 5 weeks after the last LLLT treatment.
Main outcome measures were changes in the graded MGD score (grading scale, 0–4), TBUT, score on an OSDI questionnaire, and lissamine green staining. We found significant improvements in the mean OSDI score (P = .002), MGD grading (P < .001), TBUT (P < .001), and nasal and temporal staining with lissamine green (P < .02) after LLLT. The MGD grade decreased by 1 or more in 36 of 50 eyes (72%) and by 2 or more in 16 of 50 eyes (32%). No ocular or facial adverse events or side effects related to treatment were observed.
The application of LLLT over a short series of sessions for the treatment of MGD appeared to benefit a majority of patients.
CANDIDATE SELECTION AND TREATMENT ALGORITHM
I think of LLLT as an introductory treatment. The procedure can work well even for patients with severe ocular surface disease.1 I frequently use LLLT as an introductory tool, however, with or without IPL or a treatment such as BlephEx (BlephEx), Ab Max (Myco), or the NuLids System (NuSight Medical) when anterior lid disease or blepharitis is present.
LLLT may be administered at any point in the DED algorithm, but I also use this therapy before refractive surgery and refractive cataract surgery. I find that the procedure can improve refractive measurements before PRK and LASIK and keratometry and biometry measurements before cataract surgery in patients with DED.
I have found this procedure to be beneficial for the treatment of chalazia and hordeola. Since beginning to perform LLLT 4 years ago, I have treated only two patients for acute or chronic chalazia and hordeola surgically since acquiring the Equinox. LLLT is an excellent option for children who have multiple chalazia—much more effective than surgical or systemic antibiotic therapy.5
THE PROCEDURE
During the LLLT procedure, a mask is applied for 15 minutes (Figure 2). I like to perform MGE after each treatment, but some physicians do not. I administer a fixed combination of a corticosteroid and antibiotic (typically prednisone and gatifloxacin) after LLLT and MGE because expressing long-standing, inspissated meibum can produce inflammation. Systemic doxycycline 100 mg by mouth twice per day for 2 weeks is used for most patients based on indications.
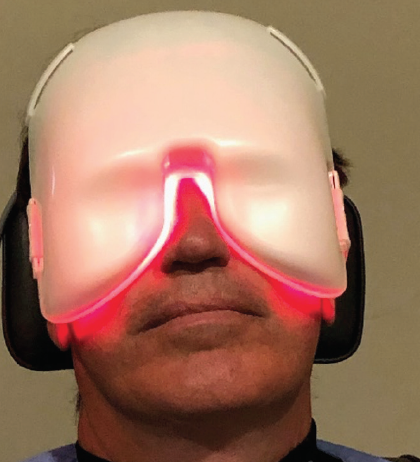
Figure 2. The mask application during LLLT.
I like LLLT because it is efficacious and easy to perform. Further, unlike IPL, LLLT can be effective regardless of the patient’s skin color, and it can be repeated if patients do not experience an effect with a single treatment. Because the heat is endogenous, there are no thermal risks for the patient.
The severity of DED6 and the patient’s treatment history dictate whether LLLT will be combined with IPL. A majority of patients, however, have been shown to experience an improvement in both the signs and the symptoms of DED even when LLLT is combined only with topical drops and systemic antibiotics such as doxycycline, as mentioned earlier.4
POTENTIAL DRAWBACKS
A reported 14% (combined LLLT and IPL) to 28% (LLLT alone) of patients did not respond to treatment initially.1,4 These statistics, however, are from patients in the initial studies in whom multiple other interventions such as topical therapy, systemic therapy, and MGD treatment (eg, LipiFlow Thermal Pulsation System [Johnson & Johnson Vision], iLux [Alcon], BlephEx) had failed. The patients from the aforementioned studies were therefore not similar to those who typically present for routine clinic.
FUTURE DIRECTIONS
One area of research that is of interest is the use of LLLT to manage DED in young patients. DED has become common in this population because of increased screen time.7 LLLT works well as initial therapy for DED, and in my experience, it is well tolerated by patients of all ages.
A controlled prospective trial comparing LLLT alone and in combination with IPL before refractive surgery and refractive cataract surgery is currently in the works. As the number of patients with DED presenting for surgery increases, I am using LLLT more often to improve the accuracy of biometry before refractive cataract surgery and to improve the manifest refraction and diagnostic accuracy with instruments such as the WaveLight Topolyzer Vario diagnostic device (Alcon) and Phorcides Analytic Engine (Phorcides) before laser vision correction.
My patients are tolerating LLLT treatment without incident, and my staff can perform this procedure with the peripheral supervision of a doctor.
1. Stonecipher K, Abell TG, Chotiner B, Chotiner E, Potvin R. Combined low level light therapy and intense pulsed light therapy for the treatment of meibomian gland dysfunction. Clin Ophthalmol. 2019;13:993-999
2. Kim WS, Calderhead RG. Is light-emitting diode phototherapy (LED-LLLT) really effective? Laser Ther. 2011;20(3):205-215.
3. Pult H. Low level light in the treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2020;61(7):99.
4. Stonecipher K, Komm C, Potvin R. Low level light therapy as an adjunct treatment for meibomian gland dysfunction. Acta Scientific Ophthalmology. 2020;3(11):13-18.
5. Stonecipher K, Potvin R. Low level light therapy for the treatment of recalcitrant chalazia: a sample case summary. Clin Ophthalmol. 2019;13:1727-1733.
6. Behrens A, Doyle JJ, Stern L, et al; Dysfunctional Tear Syndrome Study Group. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25(8):900-907.
7. Gupta PK, Stevens MN, Kashyap N, Priestley Y. Prevalence of meibomian gland atrophy in a pediatric population. Cornea. 2018;37(4):426-430.