

Glaucoma is a leading cause of irreversible blindness, and the number of patients affected by this disease is expected to increase rapidly as the population ages.1-3 It therefore should come as no surprise that an estimated 20% of patients undergoing cataract surgery have coexisting glaucoma.4 Cataract surgery on patients with advanced glaucoma can challenge even the most experienced surgeons. This article shares advice for maximizing outcomes.
SETTING EXPECTATIONS
Deciding when to proceed with cataract surgery on patients who have advanced glaucoma can be difficult. The decision requires weighing the potential for improved vision against the possibilities of further optic nerve damage and compromise of a functioning filtering bleb. Although it is reasonable to consider these risks, evidence shows that patients with advanced optic nerve cupping or fixation-threatening disease can experience a significant improvement in both vision and quality of life after cataract removal.5
Clear communication with patients is vital in this situation. Informed consent should include a thorough explanation of the potential benefits of cataract surgery and stress the ultimate limitations because of preexisting visual field loss. Additionally, it is important for the cataract surgeon to address the risks of postoperative IOP spikes and bleb failure for patients with a history of filtering surgery. Clearly outlining reasonable expectations, limitations, and risks before cataract surgery allows patients to have reasonable expectations regarding outcomes and a full understanding of the complications that may arise.
STANDALONE VERSUS COMBINED SURGERY
A major factor in surgical planning is whether the glaucoma is well controlled. In patients with elevated IOP or progressive nerve damage, the control of glaucomatous disease is the top priority.
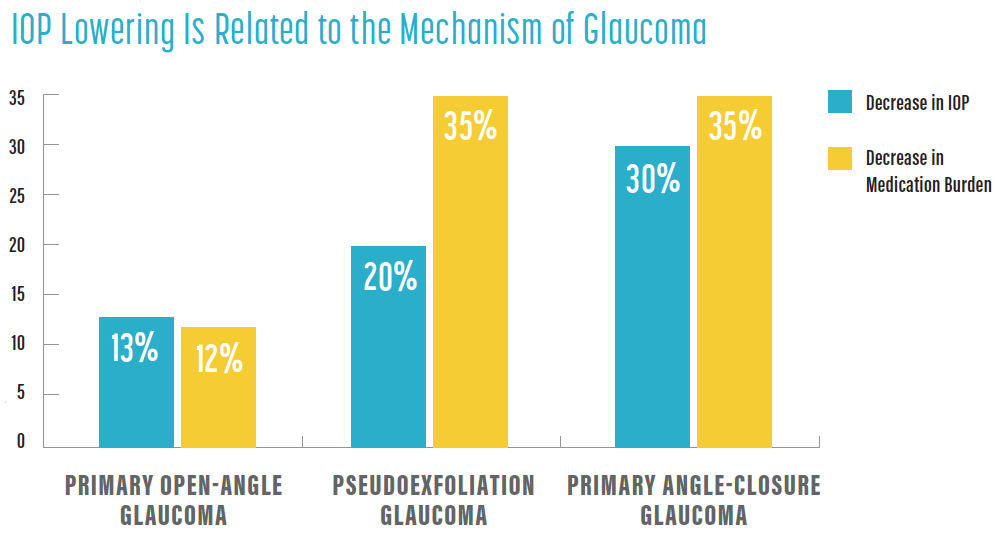
An area of interest is the ability of standalone cataract surgery to lower IOP. A review of trials to date found variable reductions in IOP and medication burden after cataract removal alone. The degree of IOP lowering appeared to be related to the mechanism of glaucoma; patients with primary open-angle glaucoma, pseudoexfoliation glaucoma, and primary angle-closure glaucoma experienced decreases in IOP and medication burden of 13% and 12%, 20% and 35%, and 30% and 35%, respectively (see IOP Lowering Is Related to the Mechanism of Glaucoma).6 Although these data are encouraging, disease severity varied among these patients, and they were treated with relatively few preoperative medications.
In a study of patients with poorly controlled glaucoma and advanced field loss, the IOP-lowering ability of standalone cataract surgery was far less certain, and many of the patients had higher IOPs after cataract removal.7 It is therefore advisable to consider a combined or staged procedure in these patients instead of relying on cataract surgery alone to reduce IOP, especially if the glaucoma is progressing.
MIGS is typically reserved for patients with mild disease, but interest is growing in the utility of this minimally invasive group of procedures for patients with advanced glaucoma. Although MIGS may be considered in patients with stable disease to decrease their medication burden, filtering surgery remains the standard of care for IOP lowering in patients with uncontrolled advanced glaucoma. Combining cataract surgery and filtering surgery offers the benefit of a single trip to the OR and the option of placing the tube in the sulcus, but the filter may fail because of vitreous loss or increased postoperative inflammation. Performing filtering surgery as a staged procedure before cataract extraction allows the bleb to mature and may limit the potential for complications. It will also, however, almost certainly accelerate the cataract, and the bleb may fail after cataract extraction. The decision between combined and staged surgery must therefore be made on a case-by-case basis.
PREOPERATIVE CONSIDERATIONS
Several preoperative considerations can help to maximize outcomes after cataract surgery.
Assessment. A careful preoperative assessment can help minimize intraoperative surprises and maximize postoperative outcomes. In patients with a history of filtering surgery, evaluating the bleb’s location and planning incisional sites can minimize the potential for surgical trauma to the bleb. If combined surgery is planned, a temporal or superior phaco approach can be considered; the choice is often driven by patient anatomy and surgeon preference. Documentation of pupillary dilation and the presence of pseudoexfoliative material can help the surgeon to anticipate the need for expansion devices and the possibility of zonular instability. In patients with a history of intraocular surgery, an evaluation of the corneal endothelium, pachymetry, and, if warranted, an endothelial cell count can help the surgeon predict the risk of postoperative corneal edema.
Lens selection. Aspheric monofocal IOLs are an excellent choice for patients with advanced glaucoma because these lenses afford better contrast sensitivity than other conventional or multifocal IOLs. Although there is still some debate about the use of multifocal IOLs in patients who have mild glaucoma, there is a general consensus that these lenses should be avoided in patients with advanced disease. The use of the latest extended range of vision IOLs may be considered if contrast sensitivity is equivalent to that of a monofocal aspheric IOL.
Aspheric toric IOLs can be successfully implanted in patients who have advanced glaucoma. It is important, however, to consider their possible future need for trabeculectomy or a tube shunt procedure because these can be associated with significant surgically induced astigmatism.
Patients who experience hypotony after filtering surgery and require cataract extraction present unique challenges. Obtaining accurate IOL calculations can be difficult. Hypotony can cause variable astigmatism, leading to inconsistent keratometry readings. Additionally, hypotony after filtering surgery can cause the axial length to decrease.8 Further complicating matters, cataract surgery can lead to bleb failure, IOP elevation, and an increase in axial length, with a resultant myopic shift in the final refraction.9 Given these concerns, it is reasonable to consider performing biometry before standalone filtering surgery or performing surgical correction of hypotony before cataract surgery.
intraoperative CONSIDERATIONS
Diligent operative planning and maneuvers to minimize intraocular inflammation are key to maximizing cataract surgery outcomes in patients with advanced glaucoma. Care should be taken during the placement of the eyelid speculum to avoid pressure or manipulation at the site of an existing bleb. Incisions should be made away from the bleb. In patients with functioning trabeculectomies or tube shunts, it is important to adjust phaco fluidics to ensure maintenance of the anterior chamber and to avoid shallowing, which can increase the risk of posterior capsular rupture.
Meticulous cortical cleanup helps to limit the degree of postoperative inflammation, and thorough OVD removal is required to minimize the risk of an IOP spike. Other options to limit IOP spikes are to administer a systemic carbonic anhydrase inhibitor intraoperatively or to instill topical glaucoma medications at the conclusion of the case.
In patients with a history of filtering surgery, it is advisable to suture the main cataract incision. A subconjunctival injection of 5-fluorouracil at the end of the case can also be considered to prevent bleb scarring.
postoperative management
Patients with advanced glaucoma require close observation after cataract surgery. In addition to the traditional 1-day follow-up appointment, it is reasonable to consider a same-day follow-up to check for an IOP spike.
Frequent visits are required to carefully monitor IOP, inflammation, and signs of early bleb failure. Studies have shown a typical, permanent rise in IOP of approximately 2 mm Hg after cataract surgery in eyes that have functioning trabeculectomies.10-12
Early intervention with increased steroid dosing, a 5-fluorouracil injection, or even needling can mitigate the risk of bleb failure.
CONCLUSION
Most patients with advanced glaucoma experience an improvement in vision and overall quality of life after cataract surgery. In our experience, they are some of the happiest postoperative patients because they appreciate not only an improvement in vision but also the care and effort that were required to achieve the best outcome.
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262-267.
2. Varma R, Lee PP, Goldberg I, Kotak S. An assessment of the health and economic burdens of glaucoma. Am J Ophthalmol. 2011;152(4):515-522.
3. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-2090.
4. Tseng VL, Yu F, Lum F, Coleman AL. Risk of fractures following cataract surgery in Medicare beneficiaries. JAMA. 2012;308(5):493-501.
5. Xu X, Sun Q, Ma YY, Zou HD. Vision-related quality of life outcomes of cataract surgery in advanced glaucoma patients. J Glaucoma. 2016;25(1):e5-11.
6. Chen PP, Lin SC, Junk AK, Radhakrishnan S, Singh K, Chen TC. The effect of phacoemulsification on intraocular pressure in glaucoma patients: a report by the American Academy of Ophthalmology. Ophthalmology. 2015;122(7):1294-1307.
7. Bojikian KD, Chen PP. Intraocular pressure after phacoemulsification in open-angle glaucoma patients with uncontrolled or marginally controlled glaucoma and/or with severe visual field loss. J Glaucoma. 2018;27(2):108-114.
8. Francis BA, Wang M, Lei H, et al. Changes in axial length following trabeculectomy and glaucoma drainage device surgery. Br J Ophthalmol. 2005;89(1):17-20.
9. Muallem MS, Nelson GA, Osmanovic S, Quinones R, Viana M, Edward DP. Predicted refraction versus refraction outcome in cataract surgery after trabeculectomy. J Glaucoma. 2009;18(4):284-287.
10. Manoj B, Chako D, Khan MY. Effect of extracapsular cataract extraction and phacoemulsification performed after trabeculectomy on intraocular pressure. J Cataract Refract Surg. 2000;26(1):75-78.
11. Rebolleda G, Muñoz-Negrete FJ. Phacoemulsification in eyes with functioning filtering blebs: a prospective study. Ophthalmology. 2002;109(12):2248-2255.
12. Derbolav A, Vass C, Menapace R, Schmetterer K, Wedrich A. Long-term effect of phacoemulsification on intraocular pressure after trabeculectomy. J Cataract Refract Surg. 2002;28(3):425-430.




