A Case of Customized Care
Won I. Kim, MD

A 77-year-old Black woman who was newly diagnosed with normal-tension primary open-angle glaucoma (POAG) was referred to me in May 2015. Her only recorded pretreatment IOP was 18 mm Hg OU. The referring provider had started her on latanoprost in each eye, and her IOP over three visits ranged from 13 to 15 mm Hg OU.
On my initial examination, the patient demonstrated severe cupping, with a 0.9 cup-to-disc ratio in each eye, and severe visual field (VF) loss of -30 dB (mean deviation) and -27 dB in the right and left eyes, respectively. I set an initial target IOP of 10 mm Hg OU, with an acceptable range of 8 to 12 mm Hg. BCVA was 20/70 OD and 20/50 OS. Potential acuity testing predicted 20/25 visual acuity OU if cataract surgery were performed.
I discussed the option of trabeculectomy with the patient due to the severity of her disease; however, she was reluctant to undergo this procedure once informed of the associated complication rate and rigorous postoperative care regimen. All the patient truly wanted was cataract surgery to improve her visual acuity. After an in-depth discussion, she accepted that undergoing a MIGS procedure at the time of her cataract surgery would not add much risk compared with cataract surgery alone and would likely keep postoperative care relatively easy.
The MIGS options available to me at the time were an iStent Trabecular Micro-Bypass Stent (Glaukos), excisional ablative goniotomy with a Trabectome (MicroSurgical Technology), ab interno canaloplasty (ABiC), gonioscopy-assisted transluminal trabeculotomy (GATT), and endoscopic cyclophotocoagulation. I wanted greater IOP-lowering efficacy than I thought the iStent could achieve. GATT presented too great a risk of significant postoperative hyphema that could delay visual recovery, which I felt would not be acceptable to this patient. Endoscopic cyclophotocoagulation could have increased the risk of cystoid macular edema (CME).
I therefore decided to use the Trabectome to eliminate the resistance at the trabecular meshwork (TM) in the nasal sector and to augment that with ABiC performed through the Trabectome cleft to encourage further IOP lowering by expanding the rest of Schlemm canal. I treated the right eye in July 2015, followed by the left eye in November 2015 (see Watch It Now).
Dr. Kim uses the trabectome and performs ab interno canaloplasty.
The patient’s postoperative course was excellent and exceeded my expectations. She maintained an IOP of 7 to 9 mm Hg OD over many visits, from 1 month postoperative to past year 2 postoperative off all medications. She maintained an IOP of 7 to 11 mm Hg OS over the same time frame, also off all medication. In June 2018, IOP was recorded as 12 mm Hg OD and 14 mm Hg OS, and I reinitiated latanoprost in each eye. Since then, her IOP over six office visits has remained between 7 and 11 mm Hg OU, up to the most recent office visit in December 2019.
This case produced results that defy conventional wisdom regarding the degree of IOP lowering that can be achieved with MIGS, and it is also notable for the longevity and durability of its effects, lasting more than 4 years. This is not to suggest that MIGS procedures will always produce these kinds of results. Such results, however, are within the realm of possibility, and combining MIGS with different and complementary mechanisms of action may improve the odds of achieving outcomes like these.
In my opinion, it is not out of the question to consider MIGS procedures as an initial surgical intervention, even in patients with severe VF loss and advanced disease. MIGS should be strongly considered as an alternative to traditional glaucoma filtration surgery, especially when performed in conjunction with phaco cataract surgery.
Sparing Limited Remaining Conjunctiva in Severe Disease
Mazeyar Saboori, MD
A 65-year-old Black man with a history of advanced glaucoma came to me for a second opinion. He had a 15-year history of POAG that was controlled medically before he was diagnosed with anterior scleritis and macular edema in both eyes, which necessitated treatment with oral steroids and methotrexate as well as topical steroids. His glaucoma became uncontrolled, and he underwent placement of a Baerveldt 350 glaucoma implant (Johnson & Johnson Vision) in the right eye and cataract extraction and Ahmed Glaucoma Valve (New World Medical) placement in the left eye.
At the time he came to see me, BCVA was 20/30 OD and 20/50 OS, and central corneal thickness was 480 µm OD and 494 µm OS. IOP was 20 mm Hg OD and 9 mm Hg OS on travoprost (Travatan, Alcon), brinzolamide-brimonidine (Simbrinza, Novartis), and timolol in both eyes.
An examination of the right eye was remarkable for inferior scleral injection, a superiorly placed Baerveldt tube shunt (encompassing the superotemporal and superonasal quadrants), 2+ nuclear sclerotic and 1+ posterior subcapsular cataract, and a cup-to-disc ratio of 0.85. The left eye was notable for a superotemporal Ahmed valve, 0.9 cup-to-disc ratio, and macular edema. The patient was administering difluprednate ophthalmic emulsion 0.05% (Durezol, Novartis) in each eye three times a day and oral methotrexate.
Gonioscopy was open to the scleral spur in each eye with a few areas of pinpoint peripheral anterior synechiae inferiorly in the right eye; of note, the tube entered the eye just anterior to the TM in the right eye. VF testing and OCT showed advanced loss in both eyes (Figures 1 and 2).
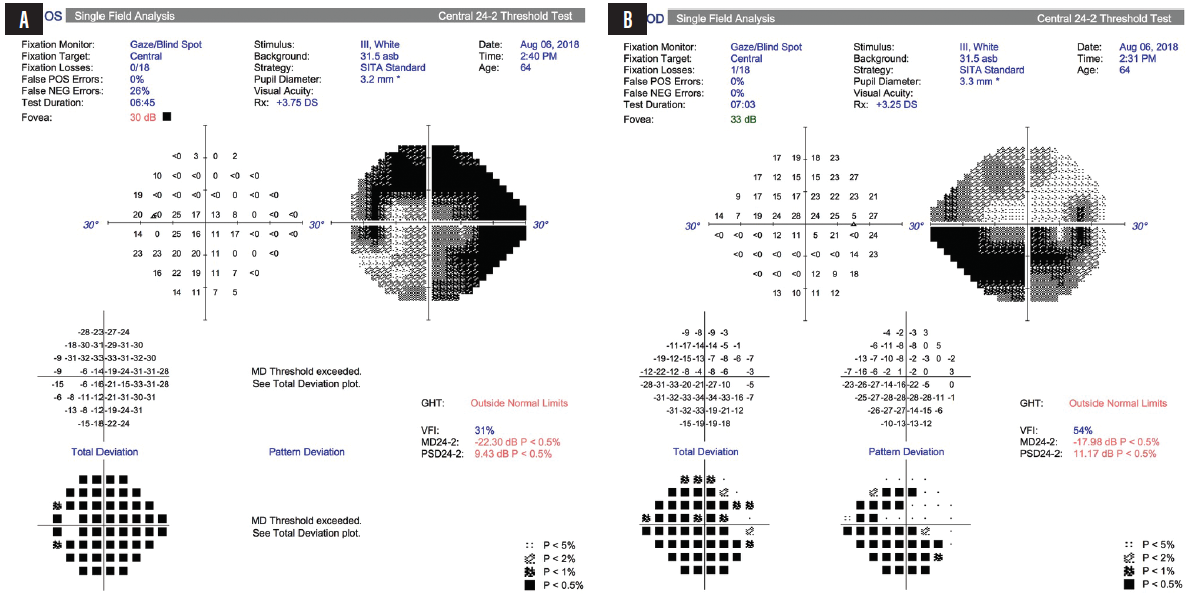
Figure 1. Advanced VF loss was observed in the patient’s left (A) and right (B) eyes.
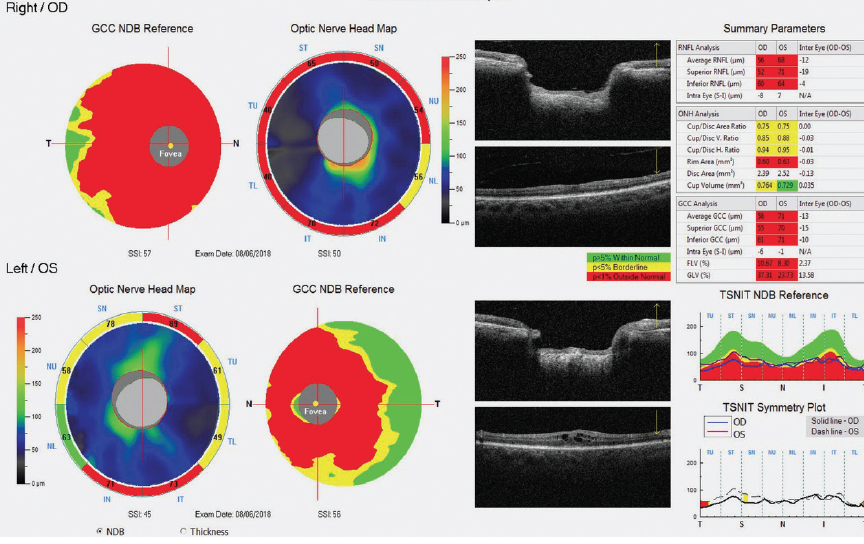
Figure 2. OCT optic nerve head maps and ganglion cell complex thickness maps showed advanced damage to the retinal nerve fiber layer and ganglion cell complex in both eyes.
Figures 1 and 2 courtesy of Mazeyar Saboori, MD
At this time, the patient was started on rituximab by his rheumatologist in an effort to decrease his need for topical steroids. However, the discontinuation of difluprednate ophthalmic emulsion 0.05% and the addition of netarsudil ophthalmic solution 0.02% (Rhopressa, Aerie Pharmaceuticals) failed to decrease his IOP. Given the patient’s visually significant cataract and inadequately controlled severe glaucoma, a decision was made to proceed with further glaucoma surgery combined with cataract extraction. Due to the placement of the Baerveldt tube shunt in both quadrants superiorly, there was no more conjunctival real estate for additional filtering surgeries superiorly.
The patient underwent cataract surgery with synechiolysis and 360º of GATT using the iTrack microcatheter (Nova Eye Medical). Cannulation of Schlemm canal was performed successfully for 360º without obstruction from the tube shunt due to its anterior placement. Viscodilation was performed while the catheter was advanced. The anterior chamber was left 50% filled with a cohesive OVD at the end of the surgery.
On postoperative day 1, IOP was 15 mm Hg, and visual acuity was 20/100 due to the presence of microhyphema. All glaucoma drops except twice-daily pilocarpine were withheld in the operative eye, and the patient was started on difluprednate ophthalmic emulsion 0.05% four times daily due to his history of uveitis. At 1 week postoperative, his IOP measured 12 mm Hg, and vision had improved to 20/40. The difluprednate ophthalmic emulsion 0.05% was tapered to once daily over the course of 2 months, and the patient experienced no steroid response during this time.
At his most recent visit, at 15 months postoperative, the difluprednate ophthalmic emulsion 0.05% has been tapered off in both eyes, with no recurrence of scleritis or CME. UCVA was 20/30 OD and 20/40 OS, with an IOP of 8 mm Hg OD and 10 mm Hg OS. The patient was instilling dorzolamide three times daily in the right eye, as prescribed by his uveitis specialist for treatment of CME, as well as travoprost, timolol, and brinzolamide-brimonidine in the left eye.
This was a particularly memorable case given the advanced nature of the patient’s disease and the lack of available untouched conjunctiva superiorly. In my hands, GATT has been successful for secondary OAG, such as uveitic and steroid-response glaucoma. This particular patient, however, had a long history of POAG prior to his diagnosis of uveitis and steroid use, which made the decision to proceed with GATT more difficult. As reported by Grover et al,1 GATT has a high probability of failure in eyes with severe glaucoma (mean deviation, ≥ -15.0). On the other hand, in secondary OAG, GATT is an effective tool for lowering IOP, with a reported average IOP reduction of 49.8%.1
I always have a candid discussion with my patients regarding the risk of failure of MIGS procedures and the possible need to proceed to further filtering surgeries. Thankfully, in this case, the need for additional filtering procedures has been mitigated, thanks to advances in safe and effective MIGS procedures.
1. Grover DS, Smith O, Fellman RL, Godfrey DG, Gupta A. Gonioscopy assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy–24-month follow-up. J Glaucoma. 2018;27(5):393-401.
A Blue Angle to Remember
Bac T. Nguyen, MD

Trypan blue dye has been safely used to improve visualization in cataract surgery and angle surgery through its staining of the anterior lens capsule and the TM.1-3 My most memorable MIGS case involved an angle that stained so vividly with trypan blue that it fundamentally changed how I teach MIGS to residents.
CASE PRESENTATION
A 70-year-old White woman with POAG and cataracts in both eyes presented with worsening vision that was interfering with her activities of daily living. BCVA was 20/40 OU. IOP was 12 mm Hg OD and 16 mm Hg OS on travoprost 0.04% and timolol maleate 0.5% instilled daily in both eyes. Gonioscopy showed open angles, Shaffer grade IV, with faint pigmentation in both eyes. The patient had 3+ nuclear sclerotic cataracts in both eyes. The cup-to-disc ratio was 0.6 OD and 0.8 OS. VFs were consistent with mild glaucoma in the right eye and moderate glaucoma in the left eye. The patient elected to undergo cataract extraction with IOL implantation along with the placement of a trabecular microbypass stent (Hydrus Microstent, Ivantis) in the left eye.
SURGICAL COURSE
Given the patient’s preoperative gonioscopic finding of faintly pigmented TM, I decided to use trypan blue ophthalmic solution 0.06% (Vision Blue, DORC International) to stain the TM and facilitate implantation of a Hydrus Microstent. After creating two paracenteses for bimanual surgery, I instilled 0.2 mL of preservative-free 1% lidocaine into the anterior chamber. Trypan blue was then injected to fill the anterior chamber and stain the anterior lens capsule and TM for 30 seconds before being irrigated with balanced saline solution. This was followed by successful cataract extraction using phacoemulsification.
After IOL implantation and removal of the OVD from the capsular bag complex, 0.1 mL of carbachol intraocular solution 0.01% (Miostat, Alcon) was instilled in the anterior chamber to induce miosis. This was followed by an injection of OVD to inflate the anterior chamber and nasal angle. The operating microscope was tilted 45º toward the surgeon, and the patient’s head was rotated 30º away from the surgeon to aid in visualization of the nasal angle for the MIGS procedure. A left-handed Swan Jacob gonioprism was placed on the cornea with an OVD coupling agent. The TM was readily identifiable by its bright blue color from the trypan blue staining (Figure 3).
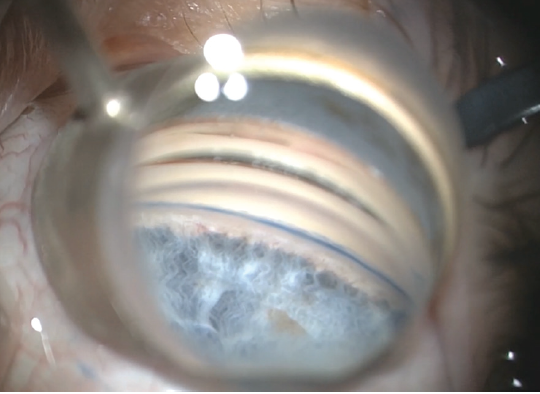
Figure 3. Intraoperative image of stained TM before Hydrus Microstent implantation.
Courtesy of Bac T. Nguyen, MD
I introduced the Hydrus Microstent injector into the anterior chamber through the temporal corneal wound. The injector cannula tip was engaged into Schlemm canal, and the Hydrus Microstent was deployed into the canal by scrolling the injector wheel forward. The injector lock was disengaged from the implant, and the injector was removed from the anterior chamber. I used a Sinskey hook to adjust the positioning of the device and then removed the remaining OVD. At 6 months postoperative, the patient’s UCVA was 20/25, and IOP was 12 mm Hg on travoprost 0.04% and timolol maleate 0.5% daily.
CONCLUSION
Although it has long been documented that trypan blue aids in enhances visualization for cataract surgery, recent advances in angle-based glaucoma surgery in adults have shown new uses for this vital dye, including staining of the TM and outlining of the collector channels.1-3 I personally had not seen a TM stain so brightly and vividly until this particular case. Most impressively, the staining persisted until the end of the procedure, despite having been applied before cataract extraction. The views seen in Figure 3 and in my video demonstration (see Watch It Now) were captured after cataract extraction and IOL implantation.
Dr. Nguyen instills trypan blue for angle visualization.
Because of this case, I changed how I teach MIGS to the residents at Baylor College of Medicine. To prepare them for MIGS, I have our senior residents tilt the microscope and rotate the patient’s head and directly visualize the angle with a Swab Jacob gonioprism during cataract surgery with and without trypan blue. This helps residents become familiar with achieving the proper positioning for angle surgery, viewing the angle anatomy, and seeing how much trypan blue can improve visualization of a lightly pigmented TM. When the residents start performing MIGS, I have them use trypan blue on their first six cases to ensure that they have the best visualization possible.
1. Melles GR, Waard PWD, Pameyer JH, et al. Trypan blue capsule staining to visualize the capsulorhexis in cataract surgery. J Cataract Refract Surg. 1999;25:7-9.
2. Grover DS, Fellman RL. Confirming and establishing patency of glaucoma drainage devices using trypan blue. J Glaucoma. 2013;22:1-2.
3. Laroche D, Nortey A, Ng C. A novel use of trypan blue during canalicular glaucoma surgery to identify aqueous outflow to episcleral and intrascleral veins. J Glaucoma. 2018;27:158-161.
Mixing MIGS During COVID-19
I. Paul Singh, MD

Glaucoma specialists today are fortunate to practice in an age with a wide variety of available treatment options. During the COVID-19 pandemic especially, I have found having access to a range of MIGS devices to be extremely valuable for addressing the individual needs and concerns of each patient who presents for glaucoma care.
This time has posed a unique set of challenges to physicians, patients, and practices alike. Over the past few months, the specific concerns on my mind have been the following:
- Identifying which patients are at risk of permanent vision loss;
- Minimizing postoperative events;
- Minimizing postoperative visits;
- Minimizing postoperative drops and compliance issues; and
- Navigating care with a leaner staff.
In light of these additional challenges, the importance of maximizing both the efficacy and safety of glaucoma treatments has been heightened. One way to achieve this goal for surgical glaucoma patients is by combining MIGS approaches with different mechanisms of action.
With conventional outflow MIGS, we do not know where the resistance to outflow is preoperatively. Is it blockage in the TM or a collapse of Schlemm canal? Are there herniations into the collector system? We have a range of individual approaches—including placement of outflow stents, dilation of the outflow system, stripping or removal of the TM—that work at different levels of the outflow system. Combining approaches potentially allows us to maximize outflow while maintaining the safety of conventional angle-based MIGS procedures.
CASE NO. 1
A 78-year-old patient presented with BCVA of 20/60 OD and 20/40 OS. The patient had ocular surface disease and a significant cataract in the right eye. Maximum IOP was 39 mm Hg OD and 33 mm Hg OS. IOP on four medications was 32 mm Hg OD and 22 mm Hg OS. Gonioscopy revealed an open angle and +2 pigmentation of the TM. The patient had a history of a failed Ex-Press Glaucoma Filtration Device (Alcon) in the right eye, rendering the conjunctiva unhealthy, as well as a history of failed selective laser trabeculoplasty in both eyes, indicating likely resistance behind the TM, either in Schlemm canal or distal channels. Corneal hysteresis was low, at around 7 OD and 8 OS (compared with a normal value of 10). This indicated that the shock-absorbing ability of the patient’s cornea might be poor, placing him at greater risk of glaucomatous progression.
Pachymetry was fairly normal, with a central corneal thickness of 523 μm OD and 534 μm OS. On OCT, however, the ganglion cell layer was almost negligible, the ganglion cell complex was wiped out, and the retinal nerve fiber layer in both eyes was extremely thin (Figure 4). The optic nerve in the right eye had a notch in the inferior rim. VF testing revealed paracentral loss with a superior defect in the right eye that was consistent with the retinal nerve fiber layer loss and disc notch. Moderate disease was also indicated in the left eye by VF testing.
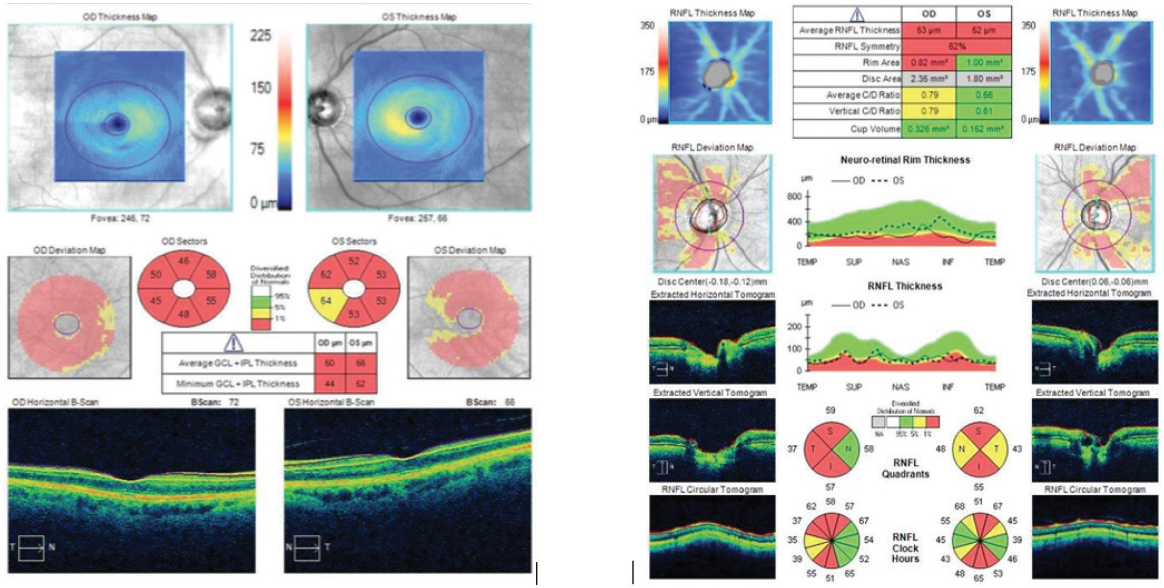
Figure 4. The patient’s ganglion cell layer was almost negligible, the ganglion cell complex was wiped out, and the retinal nerve fiber layer in both eyes was extremely thin.
Given this diagnostic picture and the patient’s concerns about coming into the office, I decided to use a combined approach, including 360º viscodilation with the Omni Surgical System (Sight Sciences), 180º goniotomy, implantation of a Hydrus Microstent, and cataract extraction (see Watch It Now). With the Omni device, I engaged the TM at about a 45º angle and advanced the nylon thread 180º in one direction. As the thread was retracted, OVD was released to dilate the canal and distal channels. I then performed the same technique to address the other 180º, but after viscodilation, I rethreaded the canal and then performed a 180º goniotomy. Next, addressing the part of the angle that was not cut, I placed a Hydrus stent, which not only bypasses the TM but also scaffolds Schlemm canal. Performing viscodilation first enables the implant to slip in beautifully.
Dr. Singh performs combination surgery with Omni and Hydrus devices.
Dr. Singh performs combination surgery with Omni and Hydrus devices.
In this case, I was able to maximize distal outflow via viscodilation, cut the TM about 180º, and, in the other 180º, place a scaffolding device to maximize outflow through the conventional pathway. On postoperative day 1, IOP was 18 mm Hg. At postoperative month 1, IOP was 14 mm Hg, and UCVA was 20/30 on brimonidine-timolol only. For this 78-year-old patient with an IOP in the 30s on four medications, by mixing MIGS procedures, I was able to maximize efficacy, decrease IOP and the number of glaucoma medications, and minimize the number of postoperative visits required compared with traditional glaucoma surgery.
CASE NO. 2
I also recently performed 360º viscodilation with concomitant placement of iStent inject (Glaukos) in a patient with open-angle glaucoma who was undergoing cataract surgery. This 68-year-old woman had mild POAG on brimonidine, timolol, and bimatoprost. She had preoperative IOPs in the low 20s with some fluctuation. She had documented progressive VF loss and a worsening cataract. Central corneal thickness was near 550 μm OU, and corneal hysteresis was around 9 OU. The patient admitted to being noncompliant with her medications.
As shown in my second video, once the patient’s cataract was removed, I obtained a beautiful view of the angle with a gonioprism, scored the TM with the Omni loader, and then aimed at about a 45º angle toward the posterior wall. I advanced the wheel to release the nylon thread into Schlemm canal. Once the wheel stopped, I reversed the wheel to retract the thread and release OVD. Going back the other way, I also engaged at about a 45º angle, advanced the wheel to release the nylon thread into Schlemm canal for the other 180º, and then retracted the wheel and nylon thread while OVD was released. This maneuver helped to dilate Schlemm canal and the distal channels.
Next, I placed the first iStent inject as perpendicular to the TM as possible while dimpling down to ensure optimal placement. About 2 clock hours away, I placed the second iStent inject. One must be careful not to dimple too much because the canal has been dilated and there is a risk of overimplantation. During irrigation and aspiration, a nice fluid wave appeared near the site of the implants, verifying that outflow had increased. After removing the OVD, I hydrated the wounds and ensured that the pressure was high enough (in the mid- to upper teens) to prevent blood reflux.
The patient’s vision improved quickly, from 20/30 on postoperative day 1 to 20/20 at postoperative week 1. IOP was 15 mm Hg on postoperative day 1, 17 mm Hg at postoperative week 1, and 16 mm Hg at postoperative month 1 off all medication. No additional or unscheduled visits were required.
A patient administering more than two or three medications, even with mild disease, is one for whom it may make sense to combine mechanisms to maximize outflow yet save the TM for a future procedure such as selective laser trabeculoplasty or a cutting/stripping procedure as needed.
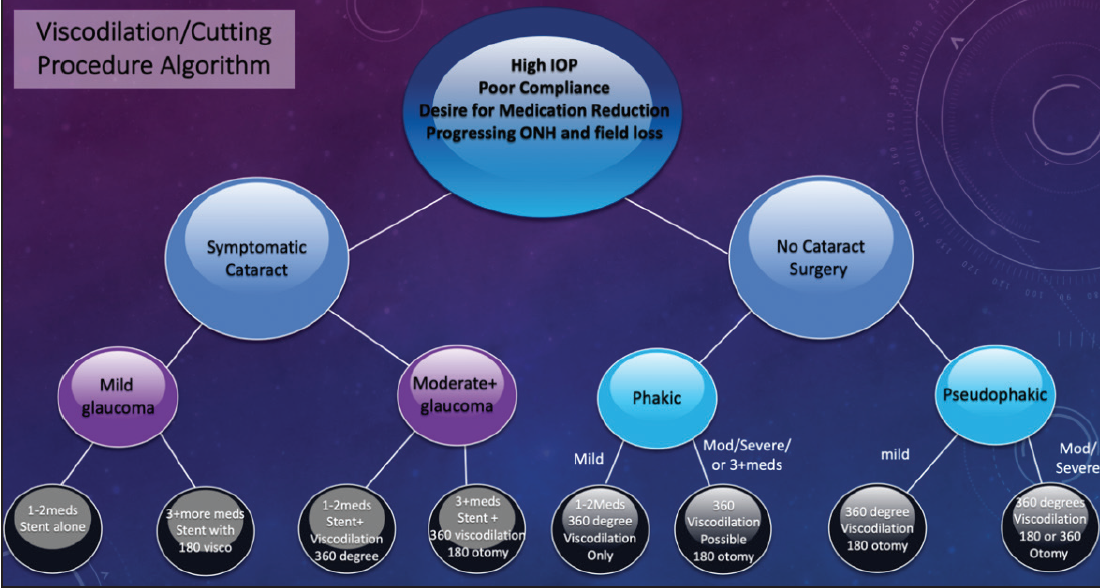
Figure 5. Dr. Singh’s treatment algorithm for mixing MIGS procedures.
Figures 4 and 5 courtesy of I. Paul Singh, MD
CONCLUSION
Mixing MIGS procedures can be extremely valuable in certain cases. Figure 5 shows my go-to algorithm for working through this decision-making process in order to identify the surgical approach that achieves optimal safety and efficacy for each patient.
*The views expressed in this article are those of the author and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or US Government.




