Evolving Administration Technique for Dexycu
By Robert J. Weinstock, MD; and Robert H. Osher, MD


Ophthalmic steroids are a mainstay of postoperative cataract surgery care, and topical options have evolved over the years. One downside shared by all topical steroids, however, is patient compliance. Success with topical steroids requires that patients have good memory and coordination and can afford the drugs. If patients do not fill their prescriptions or administer their drops correctly, they are at risk of pain, inflammation, and in some cases significant complications.
We can potentially eliminate these worries by delivering a steroid medication at the end of surgery in depot form. The advantages of this approach include reducing inflammation by placing the drug in direct proximity to the inflamed tissues and eliminating the aforementioned patient variables.
Dexamethasone intraocular suspension 9% (Dexycu, EyePoint Pharmaceuticals) is a sustained-release formulation of the familiar steroid that is administered into the posterior chamber at the end of ocular surgery. It is effective in managing postoperative inflammation.1,2
EARLY EXPERIENCE
So far, our experience with this intracameral steroid is positive. Our antiinflammatory efficacy results mirror those of the FDA trials, with early and sustained resolution of anterior chamber cell and flare. Each of us has used Dexycu in about 50 cases at the time of this writing, and no patient has reported increased pain or vision problems or required additional steroid rescue.
The product is administered as a small spherule of about 2-mm diameter. FDA labeling instructs that it is to be placed under the iris, between the anterior capsule and the posterior iris. These are slippery surfaces, and we have found in clinical practice that the droplet tends to move. It sometimes comes into the anterior chamber, landing in the inferior iridocorneal angle. This does not appear to affect efficacy, but it can be associated with transient corneal edema and may be noticed by patients.
TECHNIQUE AND TIPS
In our experience with the product, we found that it was possible to place the droplet into the capsular bag, right at the edge of the IOL optic, on the haptic-optic junction (see Dexycu Capsular Bag Administration). We have found the droplet to adhere nicely to hydrophobic and hydrophilic acrylic and silicone IOL materials, and it remains outside the visual axis.
Dexycu Capsular Bag Administration
- Remove OVD and seal incisions as you would at the end of a case
- Insert the cannula into the paracentesis, taking care to keep the chamber stable
- Advance the cannula under the anterior capsular leaflet and administer the product at the edge of the lens, at the haptic-optic junction
- Confirm that size and placement are appropriate
- Withdraw the cannula and rehydrate the incisions gently to avoid dislodging the droplet
Placement intracapsularly achieves the following:
- It prevents the drug from migrating into the anterior chamber;
- It enables the surgeon to visualize the administration and volume of drug delivered (versus beneath the iris, where visibility is limited); and
- It reduces the risk of trauma to the iris. In administering the droplet beneath the iris, it is possible to brush off pigment epithelium, which can cause pigment dispersion or transillumination defects.
In a few cases, subtle pupillary shape changes can be seen at the site of Dexycu administration. There is not enough evidence to determine whether the cause is mechanical or related to the drug; however, putting the droplet into the capsular bag allows the medication to work while minimizing risk to the iris and cornea.
It is possible to inadvertently deposit the droplet onto the center of the optic, which may affect vision for a few days as the product dissolves. If it is misplaced within or close to the visual axis, we have found it helpful to aspirate the droplet off using a 27- or 30-gauge cannula on a tuberculin syringe. A new droplet of the remaining drug can then be repositioned more peripherally. Alternatively, the droplet can be displaced using a stream of balanced saline solution to push it closer to the lens’ edge.
A small pupil may challenge visualization with either intracapsular or iris administration techniques. One tip is to ask the patient, who is under topical anesthesia, to look down to help the surgeon visualize the edge of the optic.
Regardless of where the medication is placed, surgeons will encounter a learning curve with this maneuver, which should always be the final step of surgery. After the OVD is removed and the eye sealed as though the case were complete, the supplied 25-gauge cannula or alternatively a 27- or 30-gauge cannula (which we have found easier to use) is advanced through a paracentesis, with care taken to maintain the stability of the chamber. Dexycu is then delivered as a metered dose (0.005 mL). Once the depot of drug is placed, the eye should be gently reinflated, taking care not to direct the stream of saline solution directly at the droplet.
OTHER CONSIDERATIONS
Use of the intracameral steroid requires changes to the routines of the practice and surgical staff. Patients with commercial insurance must be assessed for coverage. Knowing in advance which patients will receive Dexycu is also important because it will affect which topical drop prescriptions they are instructed to fill before surgery.
In surgery, the Dexycu vial must be shaken thoroughly to suspend the drug, and nurses and scrub technicians must familiarize themselves with use of the metered-dose syringe and administration kit.
CONCLUSION
We have seen many advantages to the intracapsular placement of a steroid at the end of cataract surgery. In our early experience, it is more reliable and straightforward for the surgeon and just as effective for patients.
1. Donnenfeld E, Holland E. Dexamethasone intracameral drug-delivery suspension for inflammation associated with cataract surgery: a randomized, placebo-controlled, phase III trial. Ophthalmology. 2018;125(6):799-806.
2. Donnenfeld ED, Solomon KD, Matossian C. Safety of IBI-10090 for inflammation associated with cataract surgery: phase 3 multicenter study. J Cataract Refract Surg. 2018;44:1236-1246.
Knowing the Three Ds Can Simplify Placement of the Dextenza Insert
By Mitchell A. Jackson, MD

Premium IOL patients expect premium visual outcomes and a premium surgical experience. Keeping pain and inflammation under control and avoiding inconvenience in the postoperative period play key roles in achieving these goals. Pharmaceutical options that incorporate innovative drug delivery techniques can help surgeons to provide these results in fewer drops.
The dexamethasone ophthalmic insert 0.4 mg (Dextenza, Ocular Therapeutix) is indicated for the treatment of ocular inflammation and pain following ophthalmic surgery. This insert can replace a tedious and complex tapered steroid drop schedule by delivering a consistent and tapered dose of the drug to the ocular surface for up to 30 days.
MY EXPERIENCE
I have been routinely using Dextenza since its launch in July 2019 in my premium IOL patients who have appropriate insurance coverage.
The insert is placed through the punctum and into the canaliculus during cataract surgery. The dexamethasone insert, conjugated with fluorescein, is easy to visualize in place using blue light with a yellow filter.
After the release of steroid to the ocular surface, the insert softens, resorbs, and exits through the nasolacrimal system. I have never had to remove one of these inserts. If this became necessary, however, such as in the case of a steroid responder, the insert can be removed by irrigating it with saline or via manual expression.
In my experience, steroids have positive effects on the ocular surface. The challenge has always been whether the patient was going to administer the eye drops as prescribed. Patient compliance is not an issue with this insert, which bathes the ocular surface with steroid continually over the course of 30 days. The insert occludes the punctum, thereby also providing benefits to the ocular surface like those we see with punctum plugs.
Patients are often pleased to avoid the added expense associated with yet another drop, saving them anywhere from $80 to $300 out of pocket.
We do not use intracameral antibiotics at our surgery center; therefore, patients must still administer postoperative antibiotic drops for 1 week, and I prescribe an NSAID to be used for 1 month postoperatively. If patients experience breakthrough inflammation or excess corneal edema postoperatively—for example, in the event of a broken capsule, a longer case requiring more phaco time, or the use of a pupil expansion device—I can have the patient add a topical steroid as needed.
TIPS FOR INSERTION
A useful way to remember the insertion process is with these three Ds: dilate the punctum, keep the insert dry, and direct the insert horizontally according to the canalicular anatomy.
Dilate. For patients who have small punctal openings, good dilation is the key to successful insertion. With a well-dilated punctum, the insert can be positioned around the 2-mm curve in the canaliculus and popped into place. I have had no trouble introducing the insert into the canaliculus, even in patients with extremely small punctae, as long as I can achieve adequate dilation.
To facilitate dilation, I use a fine-tipped dilator and, if necessary, gradually work up to a bigger tip, turning the instrument to advance dilation. Because patients are sedated, they do not feel this process.
Dry. It is important to keep the insert dry during insertion. I have my fellow or scrub technician hold a Weck-Cel sponge (Beaver-Visitec International) right below the punctum as I draw the lower eyelid in the temporal direction to avoid having fluid accumulate in the nasal punctal area. With the lower eyelid pulled temporally, nontoothed forceps are used to insert the device in a near-horizontal manner.
If the insert is two-thirds of the way in and the tail end gets wet, I can still nudge it into place. If the insert does not get at least two-thirds of the way in on the first approach, it becomes harder to position around the curve in the canaliculus.
If the front end gets wet during insertion, I turn the insert around and use the dry end to get it two-thirds of the way into the canaliculus. The last third of the wet insert can then still be coaxed into place. The manufacturer has a program that replaces any insert that gets wet prior to insertion or is accidentally dropped during insertion.
Direct. An understanding of the lacrimal system anatomy is helpful for proper insertion. There is a curve 2 mm into the canaliculus, where it bends posteriorly and medially toward the lacrimal sac. Pulling the lid laterally allows me to obtain the traction needed to maneuver the insert around that angle. Of note, the dexamethasone insert is a bit longer than standard punctum plugs.
If I am placing the insert into a right eye, I tell the patient to look to his or her right so that the eye is turned away from the nasal side where I insert the device. This helps with the insertion process and ensures additional protection for the cornea.
Some people have wide punctal openings, and the insert pops in quickly. Others may need a little more work. In the rare case when a patient does not have a punctal opening due to a cauterized punctum, I note this in the chart during preoperative evaluation. Some of my colleagues have placed inserts into the superior punctum and had good results.
PUTTING IT ALL TOGETHER
CMS has issued a permanent product-specific J-code (J1096) for Dextenza, meaning that it will be reliably reimbursed for Medicare patients. We have streamlined our approach to incorporating the insert, and we have implemented a process for confirming the patient’s insurance coverage and handling the J-code process.
With the proper infrastructure in place and an awareness of the three Ds for success, using the dexamethasone insert to replace postoperative steroid drops for my premium IOL patients has been a clear win, helping me to deliver both high patient satisfaction and good outcomes.
Durysta: Durable Glaucoma Drug Delivery Adds Important Option
By Inder Paul Singh, MD; and Steven R. Sarkisian Jr, MD

Inder Paul Singh, MD
“Doc, I just can’t remember to take my eye drops. I remember my other medications, but the eye drops seem to irritate me and don’t help me see better.”
How many times have you heard that kind of statement from your glaucoma patient? Wouldn’t it be nice not to have to spend another examination reeducating a patient on the importance of compliance? We assume that our own patients are different from those of our colleagues, but unfortunately that is not the case. In fact, two-thirds of newly treated glaucoma patients discontinue their use of drops after 1 year.1
We are often fooled by the control of IOP when we see patients because some of them are most compliant just before an appointment—they don’t want to let us down. This creates a false sense of security and further enforces the behavior of the patient and the physician.
No doubt, MIGS and selective laser trabeculoplasty (SLT) have helped providers to intervene earlier in the disease, but a number of surgeons still do not feel comfortable offering a standalone MIGS procedure, especially for young patients or those with mild glaucoma. This may be due to some surgeons’ perception that MIGS procedures lack efficacy or because it is beyond their level of comfort offering a surgical intervention.
For many of these surgeons, it may be that new extended delivery pharmaceutical options can serve as that intermediary between laser and surgery. One such option is the bimatoprost implant 10 µg (Durysta, Allergan), a dissolvable intracameral implant approved this year by the FDA.1 According to the manufacturer, the bimatoprost implant is the first FDA-approved biodegradable intracameral implant indicated to reduce IOP in patients with open-angle glaucoma or ocular hypertension via a sustained release drug delivery system.
An extended release implant could also serve to augment MIGS or traditional glaucoma surgery and provide reassurance to the surgeon and patient that the patient will have a high probability of reducing the burden of drops postoperatively. Having a durable drug delivery vehicle gives the surgeon options. If a patient needs to start medication postoperatively, the surgeon can inject the bimatoprost implant in the office. Alternatively, the implant could be administered pre- or intraoperatively at the time of the glaucoma procedure. In my opinion, this implant will help further the adoption of MIGS in general.
INTUITIVE IMPLANTATION
Implantation of the bimatoprost implant is intuitive, and the tips listed here and in the accompanying video (see Watch It Now) can be helpful to ease the learning curve for physicians who are interested in getting started with this device.
Watch It Now
Dr. Singh demonstrates his technique for insertion of the Durysta implant.
Implant it in the office setting. Most of us feel comfortable inserting a 28-gauge needle into the anterior chamber through temporal clear cornea, similar to a paracentesis. The implant can be administered in the office setting, making patient acceptance more likely compared with a procedure that requires a trip to a surgery center. As long as the surgeon has the ability to stabilize the patient’s head, maintain aseptic conditions, and use magnification, he or she can insert the implant with the patient supine or upright.
Use a second instrument. Even if it’s a cotton-tip swab, it’s important for the surgeon to use a second instrument to stabilize the eye during the procedure. There can be slight torquing of the eye as the 28-gauge needle engages the cornea, and the second instrument can help to mitigate the movement. The learning curve is fairly short, with minimal instrumentation needed.
Nail the removal of the needle. The injection involves entering the anterior chamber through temporal clear cornea parallel to the iris. Once the needle is in the chamber to a depth of 2 bevel lengths, an actuator button is pressed to release the implant, which then migrates to the inferior angle due to gravity. Removing the needle straight back out of the needle track prevents aqueous from exiting with the needle.
Select good candidates. In the studies conducted for FDA approval,2 the incidence of adverse events typically associated with topical prostaglandin use—orbital fat pad loss, lash growth, lid changes, and hyperemia—was lower in eyes treated with the implant than in eyes treated with topical bimatoprost. Therefore, any patient who complains of hyperemia or other adverse events with topical medications could be a good candidate for the implant.
Consider additional ways to enhance safety. There were no cases of endophthalmitis in the clinical trials. I recommend using a povidone-iodine prep, a speculum, and treating underlying meibomian gland dysfunction before the procedure. One can choose to place an antibiotic before and after the procedure depending on comfort level.
GOOD NEWS
What is exciting about the approval of the bimatoprost implant is the potential that consistent and continuous 24-hour intracameral delivery of bimatoprost will help to remodel the trabecular meshwork and ciliary body. In phase 1/2 trials, although the implant was designed to release medication for 4 to 6 months, a single administration of the implant controlled IOP in 40% of patients for up to 12 months and in 28% for up to 24 months.2
The potential for a longer duration of effect is key because the current FDA-approved labeling for the implant is for a single administration only. I will be using the coming months to gain experience with the implant and knowledge of which patients end up having a longer duration than expected. This experience will help guide me to set follow-up schedules once multiple dosing is, hopefully, approved. At this time, I expect to follow patients as I normally would see them, depending on the severity of their glaucoma.
The good news is that Durysta already has a miscellaneous J-code (J3490) and that it falls under Medicare Part B, not Part D. This means we will not have to wait years to gain reimbursement for the medication. Part B reimbursement is also not dictated by Medicare Administrative Contractors but rather by the manufacturer, so there will not be differences in reimbursement among the different contractors across the country, unlike newly approved stents and other surgical products that are given T-codes. (For more on coding and billing with Durysta, see the Table.) For patients with Medicare fee-for-service coverage and with supplemental insurance, there will be literally no out-of-pocket expense for this implant. Therefore, Medicare patients who have difficulty obtaining glaucoma medications would be great candidates for it.
NAVIGATING WORKFLOW
Comprehensive ophthalmologists and glaucoma specialists will have to decide on the best way to integrate the implantation procedure into their office flows. Some practices may decide to create injection blocks, as we often do for laser procedures. Others may incorporate injections throughout the day as they come. This will depend on staffing, number of patients, and surgeon preferences.
The Durysta implant expands our toolbox of procedures as we aim to reduce IOP safely while maintaining a high quality of life for our glaucoma patients. I can see myself using Durysta in a variety of scenarios; for a patient after SLT who has not achieved the desired IOP and is weary of starting or adding drops; for a patient who wanted to eliminate drops but whose MIGS procedure did not achieve the goal; or for any patient who tells me he or she cannot remember to take the prescribed medication, cannot afford it, or simply cannot tolerate it. Even if I use the implant to buy some time before a surgical intervention, that is a benefit.
I believe that, with this implant, we are witnessing the introduction of a disruptive technology similar to MIGS, one that will spark even more innovation and challenge our definition of the standard of care.
1. Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53(6):S57-S68.
2. Durysta [package insert]. Allergan. 2020. https://media.allergan.com/products/durysta_pi.pdf. Accessed June 25, 2020.

Steven R. Sarkisian Jr, MD
Sustained release medications have been a holy grail long searched for in the house of medicine. Our patients groan for relief from the use of eye drops to treat their glaucoma. Issues surrounding compliance have been with us from the beginning, whether related to cost, local side effects, forgetfulness, or difficulty administering drops correctly or at all.
Finally, we have a sustained release medication that is FDA-approved and indicated to lower IOP. The bimatoprost implant 10 µg is a polymer-based, porous microcapsule impregnated with bimatoprost, a familiar prostaglandin analogue.1 I was among the first in the country to use the implant on the first day it was available, in June, and I am happy to share my experience here.
This biodegradable intracameral bimatoprost implant is indicated to reduce IOP in patients with open-angle glaucoma or ocular hypertension via a sustained release drug delivery system. Currently, it is indicated for only one injection per eye per patient. The implant is believed to lower IOP by increasing outflow of aqueous humor through both the trabecular meshwork (conventional) and uveoscleral (unconventional) routes. It is preloaded into a single-use applicator to facilitate injection of the rod-shaped implant directly into the anterior chamber.
Efficacy data for the implant have been well covered elsewhere; it has been demonstrated to lower IOP with efficacy similar to or better than topical bimatoprost.1
FIRST PATIENTS
My first patients to receive the implant all had previous selective laser trabeculoplasty (SLT) or were taking one to three medications. I chose patients who shared my enthusiasm for this new technology. Most of them were taking a prostaglandin analogue, which I stopped after administration of the implant. I would be comfortable, however, implanting it in eyes that were naïve to drops and/or had a history of SLT only.
For months, I had been compiling a list of patients who were interested in the implant and fit the profile just described. The list included many patients who have responded to but were intolerant of topical medications. None of these first patients had very high IOPs or were candidates for glaucoma surgery. I presented the implant as the exciting opportunity that it is, without overpromising the results. I informed patients of the potential risks of any procedure in which a small incision is made.
The more ominous side effects of prostaglandins are not seen with this implant: namely, periorbital fat atrophy, dark circles under the eyes, and eyelash growth. The primary side effect is conjunctival hyperemia, which should be less than with topical administration because of the absence of topical irritation by benzalkonium chloride or other preservatives. It is amazing how many patients will jump at the chance to reduce or eliminate their use of drops.
IN-OFFICE PROCEDURE
The implantation technique for Durysta is similar to doing any paracentesis in the office (Figure 1), although it can also be done in an ambulatory surgery center (ASC). The patient is prepped with a drop of povidone-iodine solution after local proparacaine is instilled in the eye. An eyelid speculum is placed, and the preloaded inserter is set on a sterile field. The cap is removed first, followed by the safety tab after the bevel is inspected. The 28-gauge needle is then inserted through the superotemporal aspect of the cornea, pointing toward the 6:00 clock position and keeping the needle over the iris in the anterior chamber, until 2 bevel lengths are fully in the eye. The button is then firmly pressed to release the implant into the eye. The needle is removed, and the wound is checked with a cotton-tipped applicator to make certain it is watertight. Finally, the speculum is removed and a drop of antibiotic is placed in the eye.
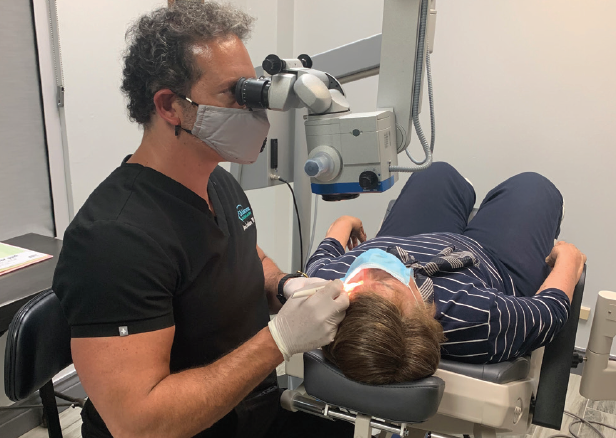
Figure 1. Dr. Sarkisian implants the Durysta device in his office using an operating microscope.
Courtesy of Steven R. Sarkisian Jr, MD
The patient is instructed to sit upright for an hour after the insertion. Patients tolerate the procedure well, and my patients have reported that there is no pain during the procedure. Some may have some local irritation after the proparacaine wears off, and they are instructed to use artificial tears as needed.
A REVOLUTION
All my first patients were pleasantly surprised by how smoothly and quickly the procedure went, and they had no complaints during or after. Although longer-term data and experience are yet to come, I predict that Durysta will revolutionize our management of glaucoma. Moreover, I predict that eye surgeons will soon be having injection clinics for glaucoma, much as we already have for macular edema. Thankfully, our lives will never be the same.
1. Durysta [package insert]. Allergan. 2020. https://media.allergan.com/products/durysta_pi.pdf. Accessed June 26, 2020.
iDose: An Intraocular Drug-Eluting Delivery Device for Glaucoma
By Mitch Ibach, OD, FAAO

Glaucoma providers are enjoying a renaissance of innovation in treatment options. One evolving category is sustained release drug delivery devices that deploy pharmacologic molecules while taking the responsibility for their instillation out of our patients’ hands.
In a 2015 study of more than 1,200 newly diagnosed glaucoma patients who were started on topical therapy, only 20% had persistently good treatment adherence at 1 year and only 15% at 4 years.1
Sustained release glaucoma medication options are expanding, but currently there is only one FDA-approved option: the bimatoprost implant Durysta. Other glaucoma drug delivery devices in phase 2 or 3 FDA trials can be subdivided into nonsurgical external modalities and surgically implanted devices. The external modalities include latanoprost microdose delivery with Optejet (Microprost, Eyenovia), latanoprost delivery via an intracameral insert (OTX-TP, Ocular Therapeutix), and latanoprost or travoprost delivery via a punctal plug (L-evolute and T-evolute, Mati Therapeutics). Surgically implanted devices in trials include two travoprost delivery vehicles, ENV515 (Envisia Therapeutics) and iDose (Glaukos).2-4
SURGICAL IMPLANT
I’ve been intimately involved with iDose as a subinvestigator in both phase 2 and 3 clinical trials. iDose is a 1.8 x 0.5 mm biocompatible titanium implant that releases a proprietary formulation of travoprost inside the anterior chamber.5 Eluting travoprost inside the eye bypasses the barrier of corneal permeability, allowing the device to release micro-amounts of the active drug molecule over time.5 The implant comprises three parts: a scleral anchor that affixes into the trabecular meshwork (TM), the body of the device that serves as the drug reservoir, and the elution membrane that titrates travoprost release (Figure 2).
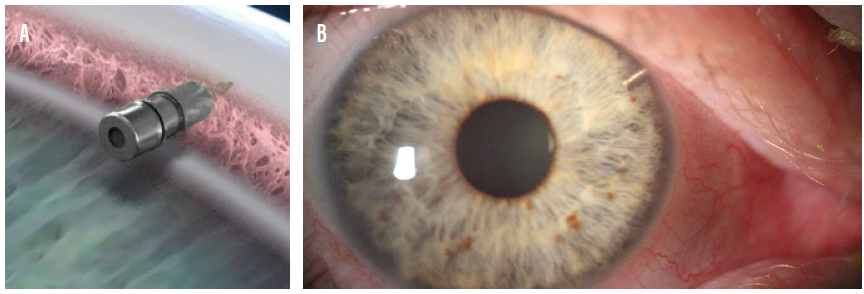
Figure 2. An illustration showing the implantation site for the iDose (A). The device implanted in an eye (B).
In our practice, implantation is performed as a standalone procedure in an ASC. After creating a 2.4-mm incision, the surgeon uses an ab-interno approach to visualize the anterior chamber angle. The scleral anchor is implanted into the TM, and the surgeon ensures secure attachment by nudging the device (see Watch It Now ).
WATCH IT NOW
The iDose is nudged to ensure secure attachment.
CLINICAL TRIALS
As previously mentioned, a phase 3 trial of iDose is under way.6 Patients in the study are randomly assigned to receive a fast-elution implant with 78 µm of travoprost, a slow-elution implant with 78 µm of travoprost, or sham surgery followed by twice-daily topical timolol maleate 0.5%.6 The primary endpoint of IOP at 3 months and secondary endpoint of IOP at 12 months are yet to be determined pending study completion, but to date these endpoints mirror closely the published endpoints from the phase 2 trial.6
The phase 2 study included 154 patients randomly assigned 1:1:1 to the fast-eluting implant, slow-eluting implant, or the control treatment of sham surgery followed by twice-daily topical timolol maleate 0.5%. All three cohorts had unmedicated baseline IOPs near 25 mm Hg. The primary endpoint was IOP reduction at 12 weeks postimplantation, with secondary endpoints including IOP at 12 months, avoidance of additional topical medications at 12 weeks, and safety outcomes.5
At the primary 12-week endpoint, the fast-eluting implant lowered IOP by 8.5 mm Hg (a 33% decrease from baseline), the slow-eluting implant lowered IOP by 8 mm Hg (a 32% decrease), and timolol lowered IOP by 7.6 mm Hg (a 30% decrease).5 This IOP reduction was stable and sustained to 12 months postimplantation (Figure 3).5 The need for additional glaucoma drops through 12 weeks was avoided by 82% of patients in both study groups and 74% in the control group.5
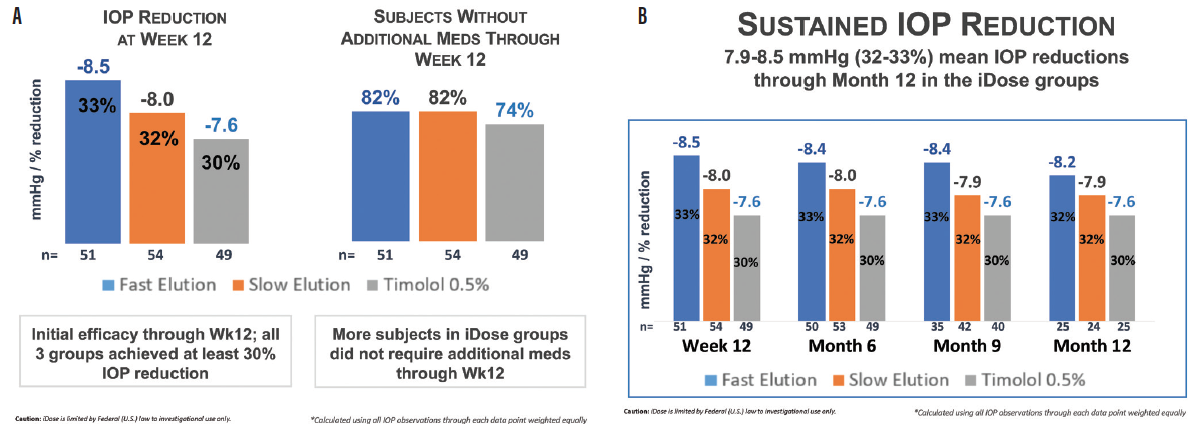
Figure 3. Phase 2 trial results at 12 weeks (A) and 1 year (B) postoperative.
Figures 2 and 3 courtesy of Mitch Ibach, OD, FAAO
Safety outcomes were favorable. There were no serious adverse events, and the mild to moderate adverse events resolved without sequelae. Through 12 weeks, there was no increased conjunctival hyperemia in either treatment arm, and travoprost was not detected in any study patients’ blood serum.5
QUESTIONS REMAIN
Even as iDose makes positive advances in completing its phase 3 clinical trial, there are still questions to be answered.
No. 1: What are our patients’ preferences regarding the mode of glaucoma treatment? In multiple studies, patient preference tilts toward drops, due to the efficacy of modern topical drop molecules coupled with their traditional status as the primary treatment option. In studies reviewing patients’ preferences in drug delivery options, extraocular approaches and devices were favored over intraocular implants.7,8 At best, 60% of patients surveyed regarding their choices for drug delivery expressed preference for an intraocular implant.9 One study showed a low of 30% preference. Higher glaucoma severity has been associated with higher drug delivery adoption across all device platforms.9
No. 2: How do eye care providers manage patients with implanted devices after the repository is empty? Possibilities include leaving the biocompatible implant in place, refilling the implant, or removing and, if needed, replacing the implant.
No. 3: What is the cost of these devices, and what is the potential reimbursement pathway? These devices are still in clinical trials, and the pathway has not been finalized. However, reimbursement will be imperative to determine true patient treatment preferences.
CONCLUSION
The paramount goals for all glaucoma drug delivery devices include improving patient compliance, increasing patient convenience, and decreasing medication side effects. The iDose travoprost implant has the potential, if approved, to check all these boxes.
1. Newman-Casey PA, Blachley T, Lee PP, Heisler M, Farris KB, Stein JD. Patterns of glaucoma medication adherence over four years of follow-up. Ophthalmology. 2015;122(10):2010-2021.
2. Schweitzer J, Ibach M. Sustained-release drug delivery: the future of glaucoma treatment? Glaucoma Today. https://glaucomatoday.com/articles/2016-nov-dec/sustained-release-drug-delivery-the-future-of-glaucoma-treatment. Accessed June 12, 2020.
3. Butchofsky B. Punctal plug delivery system. Mati Therapeutics. https://ois.net/wp-content/uploads/2019/02/GlaucomaInnovation-Mati.pdf. Accessed June 2, 2020.
4. Helzner J. Microdose latanoprost delivery set for broad patient base. Glaucoma Physician. https://www.glaucomaphysician.net/issues/2019/june-2019/microdose-latanoprost-delivery-set-for-broad-patie. Accessed June 12, 2020.
5. Ibach M. Interim results of a prospective phase II study of travoprost intraocular implants. Paper presented at: the American Academy of Optometry Annual Meeting; November 9, 2018; San Antonio, Texas.
6. Randomized study comparing two models of a travoprost intraocular implant to timolol maleate ophthalmic solution, 0.5%. ClinicalTrials.gov Identifier: NCT03519386. https://clinicaltrials.gov/ct2/show/NCT03519386. Accessed June 12, 2020.
7. SooHoo JR, Golas L, Marando CM, et al. Glaucoma patient treatment preferences. Ophthalmology. 2016;123(7):1621-1622.
8. Wang B, Lin M, Nguyen T, Turalba A. Patient attitudes toward novel glaucoma drug delivery approaches. Digit J Ophthalmol. 2018;24(2):16-23.
9. Chan HH, Wong TT, Lamoureux E, Perera S. A survey on the preference of sustained glaucoma drug delivery systems by Singaporean and Chinese Patients: a comparison between subconjunctival, intracameral, and punctal plug routes. J Glaucoma. 2015;24:485-492.
Omidria: Benefits of Drug Delivery Through the Irrigating Solution
By Steven M. Silverstein, MD, FACS

When we talk about novel routes of ophthalmic drug delivery, it is important not to forget intraoperative delivery via the irrigation solution. What could be simpler than adding a vial of medicine to the irrigation solution on the morning of surgery and not having to worry about it for the rest of the day? This is my routine practice now with phenylephrine and ketorolac intraocular solution 1%/0.3% (Omidria, Omeros). I use this fixed combination in virtually 100% of my cataract surgery patients.
This drug combination is indicated for maintaining pupil size by preventing intraoperative miosis and for reducing postoperative ocular pain. Beyond that, it reduces stress for the surgeon by ensuring that pupils remain dilated during surgery and by preventing intraoperative floppy iris syndrome (IFIS) in individuals taking an alpha-1 blocker.
Addition of phenylephrine/ketorolac to the irrigation solution doesn’t inconvenience the surgeon or the nursing staff and adds little, if any, time to the nursing routine. There’s no learning curve for the staff or the doctor. Anyone on the staff in a hospital-based facility or ASC can inject the small vial of medicine into the irrigation solution on the morning of surgery.
IFIS TRIAL
I have used this formulation in more than 2,000 eyes to date. I participated in the FDA clinical trials and other trials since the product’s approval. The most significant of these addressed a critical question: Does phenylephrine/ketorolac help us with our most challenging group of patients, which is those who are taking tamsulosin or similar alpha-1 blockers and are at risk for the triad commonly referred to as IFIS: pupillary billowing and flaccidity, iris prolapse, and iris miosis or constriction.
That prospective randomized study,1 conducted at the Silverstein Eye Centers, included 50 men taking tamsulosin who underwent routine cataract surgery. Half of the participants received Omidria and half did not. I was masked to which patients received the drugs.
Every surgery in that trial was recorded both from the perspective of the operating microscope and intracamerally. Using an endoscope and sophisticated software, we witnessed in vivo the behavior of the iris and measured the excursion of iris billowing and flaccidity. The study demonstrated conclusively and demonstrably by video that all three aspects of IFIS were dramatically reduced in patients who received phenylephrine/ketorolac in the irrigation solution.
PASS-THROUGH STATUS
Omidria has what’s known as pass-through status, which many people may be unfamiliar with or reluctant to employ. The drug has its own J-code (J1097), and use of the pass-through option has become much more streamlined than when it was initially introduced to us in 2014.
The billing can be done through the ASC, although some ASCs are not appropriately staffed to handle the preapproval measures that must be taken both before and after surgery to reconcile payments for the use of any pass-through product. Therefore, we do the paperwork in our practice’s billing department to take the burden off the ASC.
I noted previously that there is no clinical learning curve with this drug, but there may be a learning curve when it comes to obtaining prior approval and getting a handle on using the pass-through pathway. From that standpoint, the manufacturer can be extremely helpful. Omeros is available by phone to help with preapprovals and screenings. If a patient was approved and the medication was not reimbursed, the company has programs that can make up the difference to the facility, either paying for the drug completely, reimbursing for the difference if it was paid only partially, or replacing an unpaid-for vial with a fresh vial. (Editor’s note: For more on this subject, see “The Economics of Transitional Pass-Through Status,” pg 70.)
GETTING A HEAD START
It is important for users to remember that Omidria is not a pupillary dilating agent. Its purpose is to help reduce the constriction of pupil size and maintain adequate pupillary dilation during surgery.
The other aspect of the combination that I appreciate is the ketorolac component. At the time of surgery, it is helpful to have the NSAID bathing the uveal tissue, which is where we see the production and liberation of cyclooxygenase that is then converted to prostaglandins. The inflammation that results from activation of this pathway can lead to corneal edema, anterior chamber cell and flare, and cystoid macular edema (CME).
Having this agent present from the beginning to the end of surgery gives us a head start in curtailing the inflammatory cascade and reducing signs and symptoms of inflammation postoperatively. It allows patients to regain BCVA more rapidly and ensures a comfortable eye after surgery.
I don’t think of Omidria as something to be used only in challenging or higher-risk patients, but rather to be used in all patients. I certainly know that when I have cataract surgery, I will insist that my surgeon use Omidria in the irrigation solution.
1. Silverstein SM, Rana VK, Stephens R, et al. Effect of phenylephrine 1.0%-ketorolac 0.3% injection on tamsulosin-associated intraoperative floppy-iris syndrome [published correction appears in J Cataract Refract Surg. 2018;44(12):1537]. J Cataract Refract Surg. 2018;44(9):1103-1108.
Noninfectious Uveitis: Durable Corticosteroid Therapy With Yutiq Can Reduce Treatment Burden and Disease Recurrence
By Ashkan M. Abbey, MD

An injectable, intravitreal, sustained release fluocinolone acetonide 0.18 mg insert for intravitreal injection (Yutiq, EyePoint Pharmaceuticals) was approved by the FDA in 2018 for the treatment of noninfectious uveitis affecting the posterior segment (NIU-PS). The implant, in the form of a polyamide tube measuring 3.5 mm in length (Figure 4), is designed to deliver sustained release of fluocinolone acetonide for up to 36 months. In a phase 3 clinical trial, the implant demonstrated statistically significant reduction in clinical recurrences of NIU-PS and significant and sustained gains in visual acuity compared to sham treatment.
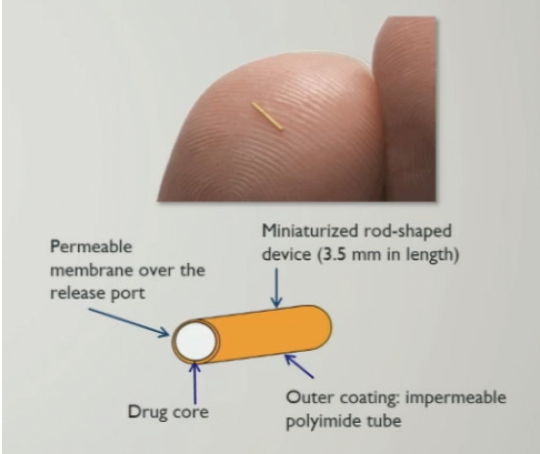
Figure 4. The Yutiq fluocinolone implant.
I have found this fluocinolone implant to be most useful in NIU-PS patients whose disease activity responds well to intravitreal steroid therapy but regularly recurs at the end of the treatment window. In my experience, many of these patients have refused systemic therapy and prefer local treatment for their NIU-PS. Oftentimes, these patients require intravitreal steroid injections every 3 to 6 months for adequate control of their inflammation and symptoms.
If a patient has not experienced an elevation of IOP after a steroid injection, it is highly unlikely that intravitreal injection of this implant will result in an unmanageable IOP spike. The acceleration of cataract formation must be discussed with patients, but most are willing to undergo cataract surgery if their NIU-PS can be successfully managed with sustained local therapy.
INJECTION TECHNIQUE
Physicians must become familiar with the injector and the steps required to prepare it, as it is quite different from previous devices we have used for intravitreal injections. For the comfort of the patient, I inject subconjunctival lidocaine inferotemporally.
After opening the packaging and removing the injector, I remove the black stopper from the plunger. The protective cap should then be removed while the injector is kept upright, to avoid having the implant fall out of the injector. Finally, the trombone wire must be removed from the needle tip while the injector is still maintained in the upright position. I prefer to use jeweler’s forceps to safely remove this wire (Figure 5). The injection can then proceed by inserting the needle at the pars plana and pushing the plunger.
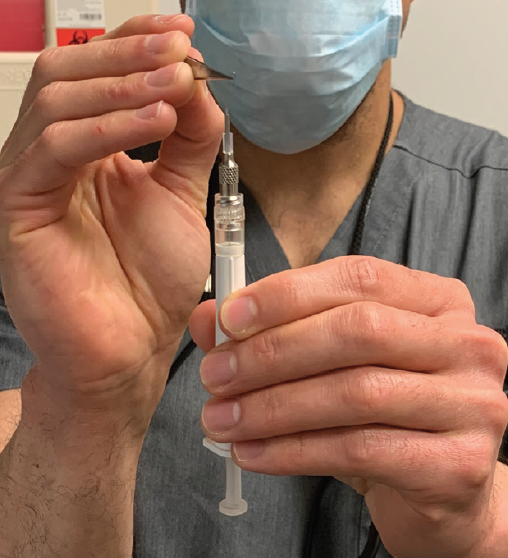
Figure 5. The trombone wire is removed with jeweler’s forceps.
CASE PRESENTATION: SARCOID UVEITIS
A 73-year-old man with sarcoidosis had been seeing me for treatment of sarcoid uveitis for the past 3 years. He had not been interested in systemic therapy for sarcoidosis and preferred local treatment for his ocular disease.
He had been visiting me every 3 months for treatment of sarcoid uveitis flares, vitritis, and CME. On each visit, disease recurrence had led to ocular disturbances and discomfort (Figure 6).
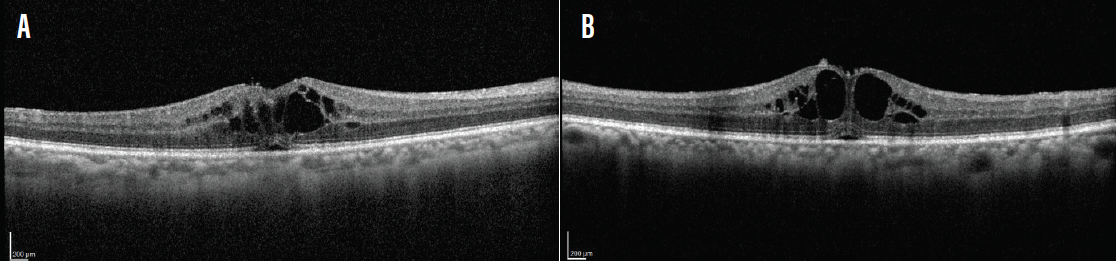
Figure 6. Regular disease recurrence in this patient led to CME in the right (A) and left (B) eyes.
For the past several years, I had treated him effectively every 3 months with the intravitreal dexamethasone implant 0.7 mg (Ozurdex, Allergan). If he missed treatment by a few weeks, however, his disease would flare. It appeared that this patient needed constant steroid therapy to keep his disease in check.
I knew that the patient’s disease responded to steroid treatment, but the treatment burden on him was high, and delays in therapy led to serious disruptions in his life. After a discussion with the patient about new therapeutic options, I decided to initiate therapy with Yutiq to reduce his treatment burden and injection frequency. Both eyes received an implant on the same day.
Six weeks later, the patient showed resolution of inflammation and CME (Figure 7). His visual acuity had improved from 20/60 to 20/20 OD and from 20/70 to 20/25 OS. He reported no visual disturbance at that visit. IOP measurements were normal, the vitritis had resolved, and the patient did not require treatment. He was instructed to return in 3 months.
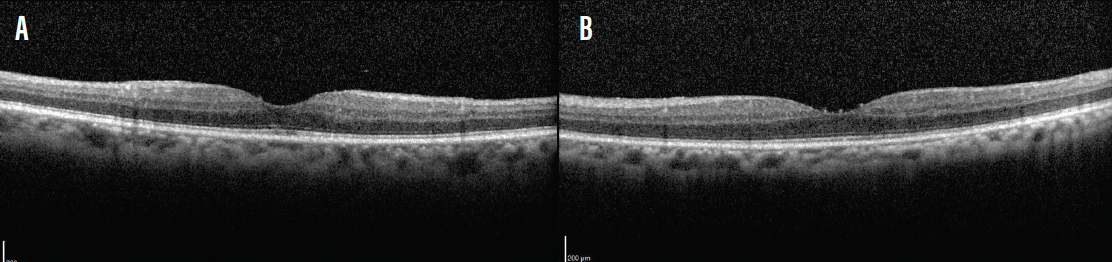
Figure 7. Six weeks after insertion of fluocinolone intravitreal implants, the patient showed resolution of inflammation and CME in the right (A) and left (B) eyes.
Figures 4–7 courtesy of Ashkan M. Abbey, MD
Upon his return, which was his most recent visit, the patient reported no discomfort, and his exam and imaging showed that his CME and disease activity were well controlled. Visual acuity was 20/20 and IOP was normal in each eye. He was asked to return again in 3 months to evaluate IOP measurements and disease activity.
DISCUSSION
Treatment with the fluocinolone implant provided the patient with considerable improvement over past regimens that required injections approximately every 3 months due to recurrent discomfort, visual disturbance, and disease activity. The fact that the patient’s disease activity has been controlled is worth highlighting, as he no longer experiences fluctuations in edema and other tissue-aggravating changes.
Yutiq is a tremendous addition to our available treatment options for NIU-PS. The sustained release of fluocinolone acetonide provides durable disease control while reducing treatment burden. This steroid delivery option will continue to play an integral role in the treatment of my patients with NIU-PS.




