CASE PRESENTATION
As routine cataract surgery becomes more efficient, extra maneuvers are required less often. Many surgeons currently use torsional or elliptical ultrasound as much as or more often than they use longitudinal ultrasound. Although torsional and elliptical ultrasound are efficient, they can encourage nuclear chips to disperse much like spittle scatters when a bulldog shakes its head. Moreover, surgeons’ instinct to laterally and thoroughly hydrate stromal incisions to close them rather than to plump the roof of these incisions until it meets the floor obscures their view of the proximal anterior chamber. For these reasons, the incidence of retained chips (Figure) remains significant.
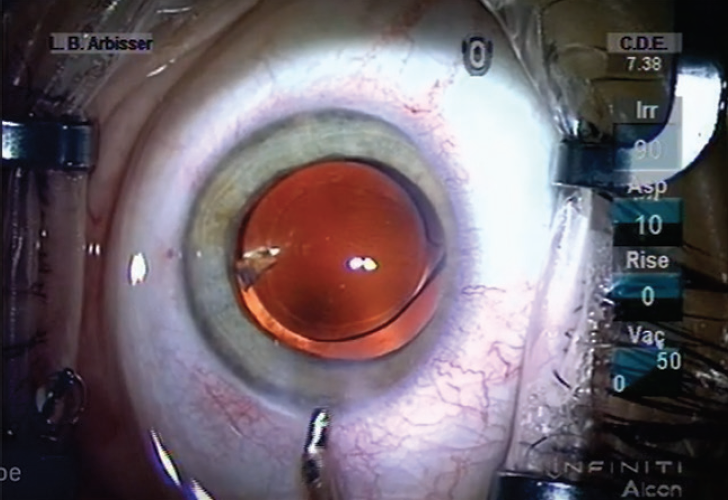
Figure. A retained nuclear chip.
How often do you observe retained nuclear chips after surgery? What measures do you take to avoid this problem, and what is your treatment paradigm when it occurs?
—Case prepared by Lisa Brothers Arbisser, MD

REBEKAH ALLEN, MD, MS
With the advent of torsional phacoemulsification, the efficiency of cataract surgery has improved, but the fluid dynamics may cause greater dispersion of nuclear chips. This in turn may increase the risk of retained fragments. I keep the aspiration rate the same even when removing the last quadrant of nucleus to avoid leaving a fragment behind. Because of the stability of current phacodynamics, this strategy does not seem to increase the risk of a capsular tear. At the end of the case, I do not hydrate the wound or the paracentesis unless it is necessary. When constructed well, incisions rarely require hydration, and this step can obscure my view of a retained chip in the anterior chamber. I also pay close attention when repressurizing the eye with a cannula at the end of surgery because placing that fluid in the anterior chamber can move retained fragments.
In my experience, retained nuclear chips are less common than retained epinuclear remnants. In the past 5 years and 6,199 cases, I have had to remove five retained nuclear fragments. Differentiating between nuclear chips and epinuclear remnants can be difficult sometimes even with gonioscopy. Retained nuclear fragments can cause localized corneal edema and increased or smoldering inflammation that does not resolve. The removal of nuclear fragments is therefore necessary.
Close observation is my preferred treatment for epinuclear fragments because the eye can resorb them. If no corneal edema or significant inflammation occurs, the eye may be watched safely for several months as the epinuclear fragment resorbs. Occasionally, a longer steroid taper is required for mild inflammation while an epinuclear remnant resorbs, but this therapy is not often necessary. It can take up to 4 months for an epinuclear chip to resorb depending on its size. If the remnant does not resorb or if it causes the aforementioned complications, I remove it surgically.

SOON-PHAIK CHEE, FRCS(G), FRCS(ED), MMED(S’PORE)
I rarely observe nuclear chips after surgery, perhaps because I do not use lateral ultrasound. During phacoemulsification, I always ensure that the fragment at the phaco tip has been removed completely and that flying chips are accounted for. During aspiration, before removing the phaco needle, I shake the eye a little to dislodge any nuclear chips. At the end of the case, I routinely search the anterior chamber for fragments. If I suspect that there is a retained fragment, I flush the anterior chamber angle with balanced saline solution. If I suspect that a fragment may be hidden in the posterior chamber, I place iris hooks with the paracenteses angled away from the iris plane so that the iris is elevated as it is retracted. Next, I direct a stream of balanced saline solution posterior to the iris, flushing through various paracenteses to dislodge the fragment.
Anterior segment OCT can be useful for imaging an occult fragment in the anterior chamber angle. Gonioscopy of the inferior angle aids visualization of a nuclear chip. I use ultrasound biomicroscopy if I suspect a posterior chamber fragment.
I perform surgical removal of the fragment on the same day I detect it to avoid its disappearance. I use automated bimanual irrigation and aspiration, which facilitates fragment capture and aspiration without a risk of repulsion by the infusion.

discussion: LISA BROTHERS ARBISSER, MD
The goal during cataract surgery is always to remove antigenic lenticular material as thoroughly as possible. To that end, surgeons must pay close attention to fragments kicked off the ultrasound tip—particularly when high ultrasound powers are used in eyes with dense nuclei—and capture those fragments upon observation.
Cortical irrigation and aspiration should proceed in an orderly manner by clock hour. I recommend starting by moving clockwise from a subincisional to a distal location (temporal surgeons operating on a left eye, for example, can start at the 3:00 clock position and move clockwise toward the 9:00 clock position) and then repeating the movement, again from a subincisional to a distal location, counterclockwise. This method offers safety because the cortex that is hardest to reach is accessed while the cul-de-sac of the bag is kept open by the bulk of cortical material. A 45º angulated I/A tip is my preference.
WATCH IT NOW
After IOL implantation, retained viscoelastic material can trap unseen nuclear fragments. OVD removal ideally involves first cleaning the posterior chamber under the IOL, while the OVD protects the endothelium. I enter the OVD-filled chamber on footpedal position zero with the I/A port opening facing sideways or upward (away from the posterior capsule). I use the I/A handpiece to nudge the IOL aside and cantilever under it with the tip without irritation (footpedal position 0). I then transition directly to aspiration (footpedal position 2) to clear the posterior chamber. Lastly, I allow the IOL to fall back into position on footpedal position zero, and proceed to clear the anterior chamber of residual contents.
If the surgeon’s automatic next step upon exiting the eye with the I/A handpiece is to aggressively hydrate the lateral sides of the stromal incision to close it, he or she will have neither a view of the proximal iris or chamber nor any certainty that all debris has been removed from between the lips of the incision or within the tunnel. For this reason, I thoroughly irrigate the clear corneal tunnel before implanting an IOL to prevent immediate chamber collapse upon withdrawal of the I/A handpiece. After evacuating the OVD, I further irrigate the tunnel and hydrate the roof of the incision rather than the lateral uncut cornea. A subtle balloting movement depressing the posterior lip of the tunnel evacuates residual material. The roof of the incision swells to meet the floor, and the incision becomes watertight if it was properly constructed. This maneuver can uncover hidden material such as a chunk or fragment of nucleus that may be hiding underneath the tunnel—the most frequent space for it to be sequestered in my experience.
This method can also remove viscoelastic material or particulate matter that may hinder the incision’s long-term closure after hydration resolves. It is important to continue to hydrate the roof of the clear corneal incision until full closure is demonstrated by a dry gutter at normotension. This will not only close the incision while the patient is on the table but also ensure that the incision will not allow the ingress of tears and infective organisms by precluding inflow once normal endothelial pump function resumes and the edema resolves or the IOP is low.
I believe that a nuclear fragment discovered postoperatively should always be removed. I suspect that there is a retained chip if the eye has unexpected or prolonged cell and flare and particularly if there is inferior corneal edema—often the presenting sign. Gonioscopy can reveal the cause of these symptoms by allowing visualization of a fragment in the inferior angle when the patient is seated upright at the slit lamp. Although a retained chip may resolve after antiinflammatory treatment, this therapy can be required for weeks, which may result in steroid-response hypertension, endothelial cell loss, and even cystoid macular edema.
Topical pilocarpine is administered preoperatively to prevent the fragment from escaping the anterior chamber. Under topical anesthesia, in the OR, an OVD is injected to compartmentalize the chip in the angle. If the fragment is small, it can be manually aspirated without turbulence using a 23-gauge cannula on a 3-mL syringe. If the fragment is large, it can be broken apart and aspirated with automated bimanual irrigation and aspiration performed through two paracenteses, leaving the main incision undisturbed. Subsequent intracameral irrigation with a preservative-free pupillary dilation agent can facilitate a thorough lavage with turbulence to detect and remove other residual fragments. In my opinion, the benefits of this quick procedure outweigh its risks.




