Rapid Relief Despite Comorbidities
By Kenneth A. Beckman, MD, FACS

A 78-year-old woman presented to our office with complaints of burning, dryness, and irritation in both eyes. She had a history of herpes simplex virus (HSV) keratitis, anterior basement membrane dystrophy (ABMD), and chronic recurrent corneal erosions in both eyes, along with severe dry eye disease (DED). She was using preservative-free artificial tears five or six times per day with little relief. She continued to have corneal erosions, which were even more difficult to manage due to her history of HSV keratitis.
On examination, severely elevated tear film osmolarity (388 mOsm/L) was detected in her worse eye. At the slit lamp, there was evidence of classic corneal ABMD with negative fluorescein staining and a very diminished tear meniscus. She was started on lifitegrast ophthalmic solution 5% (Xiidra, Novartis) b.i.d. at that visit.
At a follow-up examination 6 weeks later, she reported having experienced almost immediate improvement in her symptoms after starting the lifitegrast. Her tear film osmolarity had decreased to 289 mOsm/L in her worse eye. She stated that her dry eye symptoms had completely resolved and that she no longer needed to supplement with artificial tears.
She has now been using lifitegrast continually for almost 3 years. At each follow-up examination, her osmolarity has been either in the normal (< 300 mOsm/L) or mildly high (300–320 mOsm/L) range, with one exception. At that lone visit, one eye was moderately elevated at 330 mOsm/L. (The range for moderate elevation is 320–340 mOsm/L.) She has had no further corneal erosions or flare-ups of HSV keratitis. She also states that she still does not require artificial tears.
This case exemplifies the rapid and significant response that some patients can experience after the initiation of lifitegrast. In this case, the patient had multiple ocular surface comorbidities that were both aggravating and being aggravated by her DED. With the addition of the lifitegrast drops, she completely eliminated her dry eye symptoms and avoided recurrences of corneal erosions and HSV keratitis.
Antibiotic-Steroid Suspension to the Rescue
By Aaleya Koreishi, MD

A 70-year-old woman was referred to my practice with a recent history of trauma (a stick to the right eye). The referring physician had started the patient on moxifloxacin, and prednisolone acetate 1% was added a few days later. The patient was referred to me for consultation because of continued pain and a lack of symptomatic improvement.
On initial presentation, there was evidence of a scleral nodule with inflammation and possible infection. I presumed that a bacterial infection was present (Figure 1). Bacterial and fungal cultures were taken from the central white area and plated directly onto chocolate, blood, and Sabouraud agar. The patient was started on hourly besifloxacin 0.6% (Besivance, Bausch + Lomb), and I continued the prednisolone.
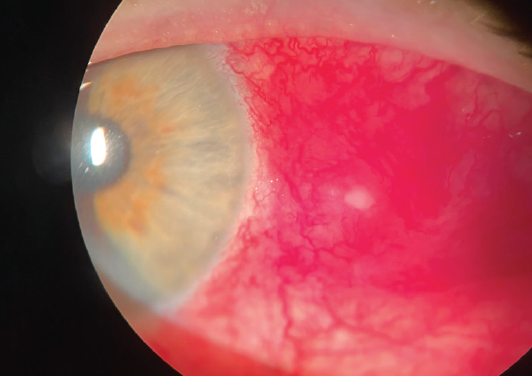
Figure 1. On initial presentation, there was evidence of a scleral nodule with inflammation and possible infection. A bacterial infection was presumed.
When she returned 5 days after her initial presentation, minimal improvement was noted, and the cultures were negative to date. Therefore, I decided to open and debride the area at the slit lamp with 0.12-mm forceps and Vannas scissors. The patient’s treatment regimen was changed at that visit to tobramycin/dexamethasone ophthalmic suspension 0.3%/0.05% with suspension technology (ST) (Tobradex ST, Eyevance Pharmaceuticals) q.i.d.
Two days after the patient started the tobramycin/dexamethasone suspension, significant improvement was noted (Figure 2). She was monitored weekly for the next 2 weeks with continued resolution. Approximately 4 weeks after initial presentation, the patient was symptom-free, and the eye showed mild scleral thinning at the site of the previous scleral abscess (Figure 3). The patient was tapered off the tobramycin/dexamethasone. Of note, the culture was held for fungus given the mechanism of injury, and the fungal culture also came back negative.
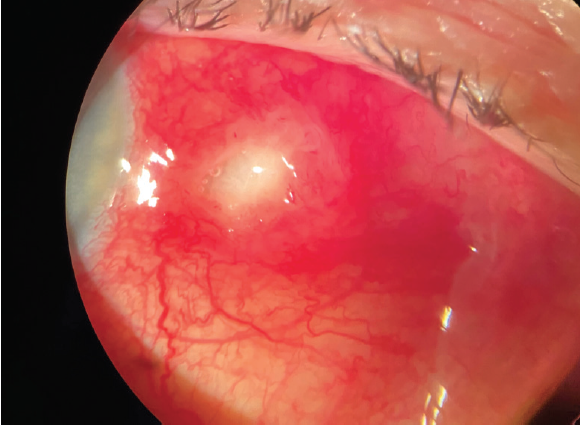
Figure 2. Two days after the patient started tobramycin/dexamethasone ST, significant improvement was noted.
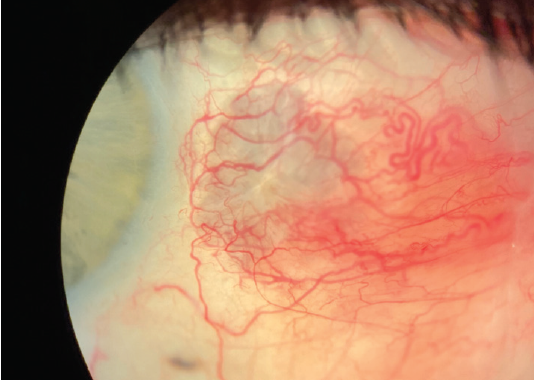
Figure 3. Approximately 4 weeks after initial presentation, the patient was symptom-free, and the eye showed mild scleral thinning at the site of the previous scleral abscess.
Figures 1–3 courtesy of Aaleya Koreishi, MD
DISCUSSION
Ocular surface inflammation with infection, or at risk for infection, may be treated with a combination antibiotic-steroid eye drop. For clinicians faced with a challenging condition for which there are several different treatment options, many factors are weighed in the decision to choose a first-line drop. Is the drop effective? Is it better than the alternatives? Are the side effects minimal? The tobramycin/dexamethasone combination with ST provides an effective treatment option in these circumstances, as demonstrated in the case report.
Tobramycin is an excellent antibiotic for gram-negative infections, but the formulation of tobramycin/dexamethasone ST with xanthan gum allows its retention on the ocular surface. This increases drug delivery, leading to more effective kill curves for traditionally hard-to-treat bacteria such as the gram-positive resistant staphylococcus aureus. This property makes tobramycin/dexamethasone ST a great choice for many ocular surface diseases.
Other disease processes that could benefit from use of tobramycin/dexamethasone ST include blepharitis, blepharokeratitis (marginal ulcers related to lid disease), and blepharoconjunctivitis. Given the bacterial proliferation, enzymatic changes, and inflammation associated with eyelid margin disease, tobramycin/dexamethasone ST can be an ideal first-line treatment choice for these and other ocular surface inflammatory processes.
Managing Ocular Issues in a Patient Taking Dupilumab
By Elizabeth T. Viriya, MD; and Francis S. Mah, MD


A 39-year-old man developed worsening ocular symptoms after 6 months of treatment with systemic dupilumab (Dupixent, Sanofi/Regeneron) for severe atopic dermatitis (AD). He presented with persistent redness around the eyes, lid crusting, severe itchiness, blurry vision, photophobia, and contact lens intolerance (Figure 4).
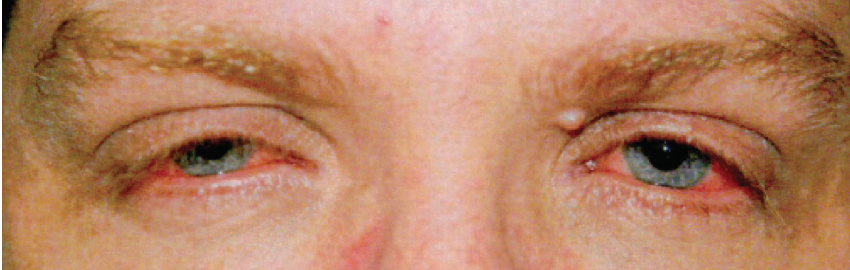
Figure 4. The patient presented with persistent redness around the eyes, lid crusting, severe itchiness, blurry vision, photophobia, and contact lens intolerance.
Courtesy of Francis S. Mah, MD
At the time of his referral to a corneal specialist, the patient was refractory to 5 months of treatment with erythromycin ophthalmic ointment, loteprednol etabonate 0.2% (Alrex, Bausch + Lomb), tacrolimus 0.03% ointment applied to the eyelids, and systemic prednisone, which had been tapered from 30 to 10 mg.
His examination was notable for rosacea facies, leathery-skin lid changes, mild keratitis, moderate hyperemia, and papillary conjunctivitis with filaments.
His AD was especially difficult to manage, so he and his dermatologist had hopes to continue the dupilumab because, according to the patient, “we finally found something that works.”
A differential diagnosis assessment was performed to distinguish between atopic blepharitis and dupilumab-induced filamentary conjunctivitis and keratitis.
Management consisted of continued dupilumab use with the addition of the following in each eye: cyclosporine ophthalmic emulsion 0.05% (Restasis, Allergan) b.i.d., loteprednol etabonate 0.38% (Lotemax SM, Bausch + Lomb) b.i.d., and loteprednol etabonate ophthalmic ointment 0.5% (Lotemax Ointment, Bausch + Lomb) q.h.s. Within 2 to 3 weeks, the filaments resolved and conjunctival injection improved from moderate to mild. Therefore, the loteprednol drops and ointment were slowly tapered while cyclosporine b.i.d. was maintained.
The patient’s symptoms resolved, and he self-discontinued the cyclosporine by mistake. When his ocular inflammation recurred, cyclosporine and a steroid bridge were used to achieve quiescence. He remained stable on topical cyclosporine 0.05% monotherapy thereafter. His condition improved so much that he returned to wearing soft contact lenses safely and comfortably.
DISCUSSION
AD is a chronic Th2-cell mediated inflammatory condition; of patients with AD, 26% to 42% will also present with atopic keratoconjunctivitis (AKC). Management of AKC includes allergen avoidance, lubrication, and steroid pulses for exacerbations. An immunopathology study of conjunctival biopsies in patients with AKC showed reductions in T cells and cytokines with topical cyclosporine A 0.05% six times daily for 2 weeks followed by q.i.d. dosing for 4 weeks.1 This regimen also improved signs and symptoms, but a rebound was observed after discontinuation.2
A new element in AD management is dupilumab, an FDA-approved biologic therapy for moderate to severe AD. This monoclonal antibody blocks receptors for IL-4 and IL-13, inhibiting Th2-mediated inflammation. This modality is efficacious for dermatitis. A review of randomized, placebo-controlled trials found a higher incidence of ocular findings, especially conjunctivitis, after dupilumab use for AD, but not in patients treated with dupilumab for asthma, eosinophilic esophagitis, or rhinosinusitis.3
Dupilumab-associated ocular findings can vary from a mild, nonspecific conjunctival hyperemia and follicular conjunctivitis to keratitis. The etiology is unknown, but the findings are associated with a history of conjunctivitis, increased immunoglobulin E or eosinophil counts, and more severe AD. The International Eczema Council does not view conjunctivitis as a contraindication to dupilumab use. It recommends comanagement with an ophthalmologist.4
Ophthalmologists have a role to play at all stages of AKC management and the dupilumab course. Prophylactically, the ocular surface can be optimized and pulsed topical steroids considered when dupilumab injections are given. Intervals between injections can be adjusted 1 to 2 weeks to reduce adverse effects. Follow-up intervals will vary, as onset can be as soon as 2 weeks or delayed by a few months. Management is supportive for mild cases. Moderate to severe stages may require a steroid bridge with long-term use of calcineurin inhibitors or lifitegrast ophthalmic solution 5%.5 Discontinuation is rarely warranted, and the washout period is prolonged at about 8 to 10 weeks.
SUMMARY
Management of AKC and the ocular adverse events induced by dupilumab requires long-term topical immunomodulation. This case highlights the utility of steroid-sparing agents, including calcineurin inhibitors such as cyclosporine, which have been effective in improving patient signs and symptoms such as conjunctivitis and keratitis.
1. Utine CA, Stern M, Akpek EK. Immunopathological features of severe chronic atopic keratoconjunctivitis and effects of topical cyclosporine treatment. Ocul Immunol Inflamm. 2019;27(7):1184-1193.
2. Akpek EK, Dart JK, Watson S, et al. A randomized trial of topical cyclosporin 0.05% in topical steroid-resistant atopic keratoconjunctivitis. Ophthalmology. 2004;111(3):476-482.
3. Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181(3):459-473.
4. Thyssen JP, de Bruin-Weller MS, Paller AS, et al. Conjunctivitis in atopic dermatitis patients with and without dupilumab therapy – International Eczema Council survey and opinion. J Eur Acad Dermatol Venereol. 2019;33(7):1224-1231.
5. Agnihotri G, Shi K, Lio PA. A clinician’s guide to the recognition and management of dupilumab-associated conjunctivitis. Drugs R D. 2019;19(4):311-318.
Antihistamine Eye Drop Can Curb Ocular Itching
By Ranjan P. Malhotra, MD, FACS

A 42-year-old woman who has been working from home due to the coronavirus pandemic presented for a telemedicine consultation with the chief complaints of irritated, red, and inflamed eyes; foreign body sensation; and excessive tearing (Figure 5). Her allergies, she explained, are considerably worse this year, likely related to attending excessive Zoom conference calls and to exercising outside instead of at her local gym.
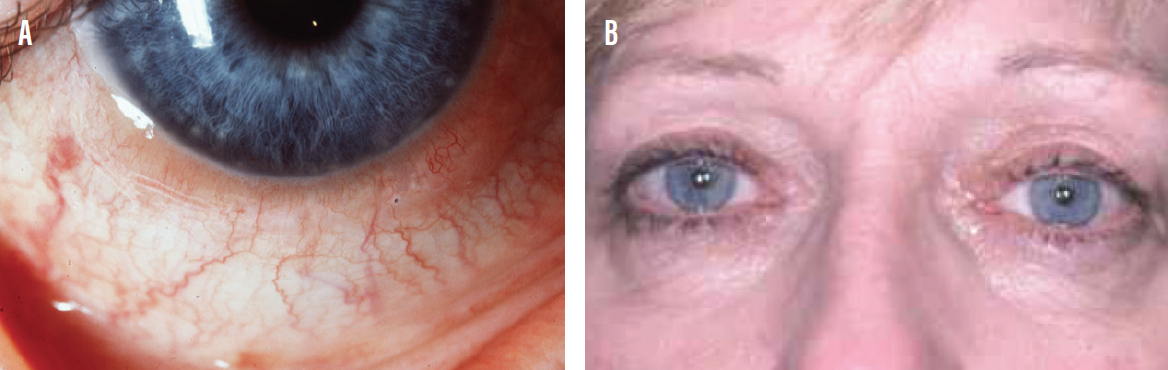
Figure 5. A 42-year-old woman presented to a telemedicine consult with allergic conjunctivitis (A, B).
Courtesy of Ranjan P. Malhotra, MD, FACS
The patient reported trying over-the-counter artificial tears with no relief of symptoms. She has used oral cetirizine HCl (Zyrtec, Johnson & Johnson) with some success in the past; however, it makes her drowsy and she wants to avoid prolonged use.
According to the patient’s chart, she also has concomitant dry eye. Rather than bringing her in for a visit, I suggested that she try cetirizine ophthalmic solution 0.24% (Zerviate, Eyevance Pharmaceuticals), instilled in the eyes b.i.d., approximately 8 hours apart. This eye drop, a histamine-1 receptor antagonist indicated for the treatment of ocular itching associated with allergic conjunctivitis, combines Hydrella, a vehicle containing two ingredients in tear lubricants (glycerin and hydroxypropyl methylcellulose), with the same antihistamine found in oral cetirizine. This vehicle combination helps to deliver the antihistamine molecule to the eye comfortably and to reduce swelling, itching, and vasodilation.1,2
At a follow-up telemedicine visit 4 weeks later, the patient reported a noticeable and almost immediate improvement in her symptoms. The inflammation and redness in her eyes was gone, and she no longer was experiencing foreign body sensation.
CONCLUSION
More than 80 million individuals in the United States alone have allergic conjunctivitis. Patients who seek relief from ocular allergies often benefit from the use of an antihistamine, but the side effect of drowsiness is not always tolerable. In those circumstances, prescribing a treatment such as cetirizine ophthalmic solution 0.24% is a good alternative. Even patients who do well with an oral antihistamine might prefer an ocular formulation that also includes two active ingredients found in tear lubricants. In the short time that this product has been available, I find myself prescribing it with increased frequency.
1. Charlesworth EN, Kagey-Sobotka A, Norman PS, Lichenstin LM. Effect of cetirizine on mast cell-mediator release and cellular traffic during the cutaneous late-phase reaction. J Allergy Clin Immunol. 1989;83(5):905-912.
2. Levi-Schaffer F, Eliashar R. Mast cell stabilizing properties of antihistamines. J Invest Dermatol. 2009;129(11):2549-2551.
Dry Eye Med Cures Cataracts?
By Darrell E. White, MD

You never know when, or how, circumstances will conspire to teach you a lesson. The recent COVID-19–related lockdown of ophthalmology practices reinforced the lessons I learned from William B. Trattler, MD, about the importance of the ocular surface in all things vision. At the same time, this experience demonstrated for me how well (and how quickly) the newest FDA-approved dry eye medication can work to alleviate the visual effects of dryness.
Shortly before we closed in March, I saw a 62-year-old woman with modest cataracts (2+ nuclear sclerosis) who was bitterly unhappy with her vision. She was especially unhappy with her symptoms while driving at night. Checking her vision confirmed both her general blur (20/30-2 OD, 20/40 OS) and her challenges with nighttime glare (brightness acuity test, 20/80 OD, 20/150 OS). On examination, I also noted moderate DED. She had 1 to 2+ corneal staining with fluorescein, and her tear breakup time was 4 seconds in each eye.
I find the concept of visually significant DED, first proposed by Starr et al in an ASCRS white paper on perioperative DED,1 to be useful. My patient clearly had DED that would have to be treated in order to obtain useful preoperative measurements, no matter what type of IOL we eventually chose.
Because her DED was moderate, I prescribed only cyclosporine ophthalmic solution 0.09% (Cequa, Sun Ophthalmics) in each eye t.i.d., along with nonpreserved artificial tears. I asked the patient to return in 2 to 3 weeks for repeat measurements. Her surgery was tentatively scheduled for 6 weeks from her original visit date, the first available OR slot at the time.
And then, like every other ophthalmology practice in America, SkyVision closed for all but emergency care. My unhappy patient was forced to wait 6 weeks for her follow-up visit, and her surgery date was cancelled. With many months of experience with this nanomicelle formulation of cyclosporine, I expected that her ocular surface would be improved enough to obtain solid measurements, and my surgical coordinator told her that we would do our best to get her on the OR schedule.
During the lockdown, the patient had not been out at night but she told her workup technician that her vision seemed to be better; she was binge-watching “Ozark” and remarked how much older Jason Bateman seemed to be looking since she started her DED drug.
You know where this is going, of course.
Her vision while wearing her 2-year-old prescription was now 20/20-2 OD and 20/20 OS. There was a single, lonely punctate spot that stained on her right cornea, and her superficial punctate keratitis was otherwise resolved in each eye. Tear breakup time was now 7 seconds in each eye. Her brightness acuity testing was also significantly improved in both eyes (20/30 OD, 20/40-2 OS).
What had been a straightforward case of treating the ocular surface for visually significant DED in the perioperative period became a reminder of how important a healthy tear film is when it comes to treating our most common presenting symptom—blurred vision.
With her surgery cancelled, we called in a 90-day supply of Cequa for our no-longer-preoperative patient and arranged a standard 3 month DED follow-up exam. I heard that she bought a pair of prescription Maui Jim sunglasses to be ready when the golf courses opened up.
1. Starr CE, Gupta PK, Farid M, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45(5):669-684.