AZR-MD-001: A Familiar Compound in a Novel Formulation
By Laura M. Periman, MD

The meibomian glands are modified sebaceous glands in the eyelid margin that are responsible for secreting meibum, the principal constituent of the outer tear film lipid layer. (This lipid layer is responsible for lubricating the ocular surface during blinking and protecting against tear evaporation.1-4) Meibomian gland dysfunction (MGD), a chronic, diffuse abnormality of the meibomian glands characterized by gland obstruction, microbiological changes, inflammation, altered meibum quality, compromised meibomian gland flow (stasis) and increased melting temperatures, and changes in the amount or quality of secreted meibum.5,6 MGD has been identified as a common component of ocular surface disease (OSD),7-11 a condition that is characterized by instability of the tear film, drying of the ocular surface, and ocular discomfort.12
Hyperkeratinization of the meibomian gland orifice and ductal epithelium, a typical finding in the obstruction component of MGD,13-15 is made up of tightly crosslinked keratin strands.16,17 MGD raises meibum melting temperature; increases meibum viscosity; and results in stasis, gland plugging, and gland obstruction. This all may ultimately lead to gland dilation, atrophy, and dropout.3,4,16-19 The understanding of MGD has expanded beyond simple obstruction, and the six interrelated mechanisms of MGD have been well described.6
Meibomian glands share strong similarities with sebaceous glands with respect to both embryologic development and structure, including the ability to undergo keratinization. Considering these similarities, the meibomian glands may be regarded as hair follicles without a hair shaft.20 In addition to being involved in diseases affecting the lid margin, the process of secretory gland hyperkeratinization plays a role in various skin disorders including acne, seborrheic dermatitis, psoriasis, and dandruff.2,21,22
Keratolytic agents that reduce hyperkeratinization of the skin and sebaceous glands have been successfully used to treat these conditions.23-25 Could a similar approach work to treat the keratinization seen in MGD?
NEW TREATMENT FOR MGD
The primary aim of treatment for MGD is to restore the normal quality, quantity, and flow of meibomian gland secretions, thereby increasing the likelihood of a healthy lipid layer and stable tear film. This can be facilitated in part by addressing the obstructing material.3 Conventional methods for removal of abnormal gland material include various mechanical (eg, Maskin Probing) and heat-based (eg, in-office thermal pulsation, warm compresses) techniques. Thermal approaches to the obstruction component of MGD treatment are effective in melting the oils but are unable to address the disulfide-bonded keratin strands in the meibum due to the high temperatures (145°C) necessary to break the bonds and solubilize the keratin strands and plugs.26 There is potential for excellent clinical synergy in addressing the keratin plugs along with the current excellent MGD therapeutic modalities.
The use of combined dermatologic-ophthalmologic approaches to treatment of MGD is on the horizon. This novel approach to MGD addresses obstruction from hyperkeratinization (in a nonthermal fashion) and the hyposecretion of meibum.27,28 Keratolytic agents that chemically, rather than thermally, break disulfide bonds hold potential for restoring meibomian gland function by targeting multiple mechanisms involved in the early stages of MGD pathogenesis. One such keratinolytic agent, selenium sulfide, can effectively treat skin disorders such as dandruff and seborrheic dermatitis.23,24
AZR-MD-001 (Azura Ophthalmics) is a novel ophthalmic formulation of selenium sulfide under development for MGD. It has a triple mechanism of action, according to data on file with Azura, to promote the breakdown of disulfide bonds in keratin, slow the production of abnormal keratin, and stimulate meibum production in vitro.
In an 18-patient company-conducted pilot study, patients received selenium sulfide in a nonoptimized formulation in one eye; the contralateral eye served as control. The study demonstrated significant improvements in tear breakup time, meibum quality, and meibomian gland secretion scores for the drug-treated eye versus the contralateral control eye, as observed by day 22 after treatment (data on file, MGSS1 Azura). Further studies are underway in the drug development process.
CONCLUSION
The clinical science behind combined dermatologic-ophthalmologic approaches to treatment of MGD is in its infancy, but a treatment such as AZR-MD-001 represents an exciting new therapeutic category in the development of strategies to address the obstruction and hyposecretion problems of MGD.
NRO-1 Could Potentially Protect, Regenerate Corneal Nerves
The first patient has been enrolled in a phase 2 clinical trial of a novel therapeutic agent that protects and regenerates corneal nerves, according to developer Neuroptika.1 This therapeutic, according to the company, has potential to protect and regenerate damaged corneal sensory nerves and to become a disease-modifying treatment. NRO-1 works to normalize the ocular surface and improve symptoms of DED.
The aim of the multicenter, randomized, double-masked, vehicle-controlled phase 2 clinical trial is to evaluate the safety and efficacy of two concentrations of NRO-1 in patients with DED. The study will enroll 120 patients with moderate to severe DED who will receive one of the two concentrations or vehicle for 28 days. Results from the phase 2 study are expected to be available in the second half of this year.
1. https://eyewire.news/articles/neuroptika-announces-enrollment-of-first-patient-in-phase-2-clinical-trial-for-dry-eye-disease/. Accessed June 24, 2020.
1. Green-Church KB, Butovich I, Willcox M, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011;52(4):1979-1993.
2. Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2:149-165.
3. Blackie CA, Korb DR, Knop E, Bedi R, Knop N, Holland EJ. Nonobvious obstructive meibomian gland dysfunction. Cornea. 2010;29(12):1333-1345.
4. Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52(4):1938-1978.
5. Nichols KK. The international workshop on meibomian gland dysfunction: Introduction. Invest Ophthalmol Vis Sci. 2011:52;1917-1921.
6. Geerling G, Baudouin C, Aragona P, et al. Emerging strategies for the diagnosis and treatment of meibomian gland dysfunction: Proceedings of the OCEAN group meeting. Ocul Surf. 2017;15(2):179-192.
7. Lemp MA. Report of the National Eye Institute/industry workshop on clinical trials in dry eyes. CLAO J. 1995;21(4):221‐232.
8. Goto E, Endo K, Suzuki A, et al. Tear evaporation dynamics in normal subjects and subjects with obstructive meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2003;44:533-539.
9. Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993;100:347-351.
10. Mathers WD, Lane JA. Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol. 1998;438:349-360.
11. Mishima S, Maurice DM. The oily layer of the tear film and evaporation from the corneal surface. Exp Eye Res. 1961;1:39-45.
12. Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52(4):1994-2005.
13. Foulks GN, Bran AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003;1:107-126.
14. Jester JV, Nicolaides N, Smith RE. Meibomian gland dysfunction. I. Keratin protein expression in normal human and rabbit meibomian glands. Invest Ophthalmol Vis Sci. 1989;30(5):927-935.
15. Jester JV, Nicolaides N, Kiss-Palvolgyi I, Smith RE. Meibomian gland dysfunction. II. The role of keratinization in a rabbit model of MGD. Invest Ophthalmol Vis Sci. 1989;30(5):936-945.
16. Ong BL, Hodson SA, Wigham T, et al. Evidence for keratin proteins in normal and abnormal human meibomian fluids. Curr Eye Res. 1991;10:1113-1119.
17. Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):2006-2049.
18. Obata H. Anatomy and histopathology of human meibomian gland. Cornea. 2002;21(7 Suppl):S70‐S74.
19. Nelson DJ, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):1930-1937.
20. Knop E, Ludescher M, Knop N. Keratinisation status and cytokeratins of the human meibomian gland epithelium. Acta Ophthalmol. 2009;87:2232.
21. Plewig G, Kligman AM. The effect of selenium sulfide on epidermal turnover of normal and dandruff scalps. J Soc Cosm Chem. 1969;20:767-775.
22. Rapaport M. A randomized, controlled clinical trial of four anti-dandruff shampoos. J Int Med Res. 1981;9(2):152-156.
23. Wong AS, Fasanaella RM, Haley LD, Marshall CL, Krehl WA. Selenium (selsun) in the treatment of marginal blepharitis. AMA Arch Ophthalmol. 1956;55(2):246-253.
24. Piérard-Franchimont C, Arrese JE, Piérard GE. Sebum flow dynamics and antidandruff shampoos. J Cosmet Sci. 1997;48:117-121.
25. Cohen PR, Anderson CA. Topical selenium sulfide for the treatment of hyperkeratosis. Dermatol Ther (Heidelb). 2018;8(4):639‐646.
26. Istrate D, Popescu C, Möller M. Non-isothermal kinetics of hard alpha-keratin thermal denaturation. Macromolecular Bioscience. 2001;9(8):805-812.
27. Akyol-Salman I, Azizi S, Mumcu U, Baykal O. Efficacy of topical N-acetylcysteine in the treatment of meibomian gland dysfunction. J Ocul Pharmacol Ther. 2010;26(4):329-333.
28. Akyol-Salman I, Azizi S, Mumcu UY, Ates O, Baykal O. Comparison of the efficacy of topical N-acetyl-cysteine and a topical steroid-antibiotic combination therapy in the treatment of meibomian gland dysfunction. J Ocul Pharmacol Ther. 2012;28(1):49-52.
KPI-121 0.25%: MPPs Helpful for Short-Term Treatment
By Preeya K. Gupta, MD

Up to 50% of the population experiences DED, and most cataract patients show signs of OSD.1,2 Any OSD, commonly DED, affects refractive measurements and must be treated proactively to achieve accurate keratorefractive and phacorefractive outcomes in cataract surgery.
As a member of the ASCRS Cornea Clinical Committee, I recently collaborated on the development of a consensus-based algorithm to guide treatment of DED preoperatively, helping to ensure accurate measurements and IOL calculations.3 Antiinflammatory agents targeting T lymphocytes in the DED inflammatory cycle comprise the approved prescription treatments. Topical ophthalmic corticosteroids used as adjunctive therapy or monotherapy provide a rapid onset of action. For patients with preoperative tear film instability or corneal staining, I often prescribe a 2-week course of topical ophthalmic corticosteroids, after which biometry and topography are repeated. Most patients tolerate topical steroids; however, clinicians must monitor for safety.
MPPS IMPROVE DRUG DISTRIBUTION
KPI-121 0.25% (Kala Pharmaceuticals), a corticosteroid formulation that employs mucus-penetrating particles (MPPs), is in development for short-term treatment of DED. KPI-121 0.25% is a nanoparticle suspension of loteprednol etabonate formulated with the company’s MPP technology.4
The nanoparticle formulation of loteprednol with a mucus-penetrating coating can improve the uniformity and distribution of loteprednol, enhancing drug delivery to ocular tissues. Loteprednol is often preferred for OSD treatment due to its rapid metabolization by cellular esterase and favorable safety profile.5
CLINICAL DEVELOPMENT
KPI-121 0.25% was evaluated in one phase 2 and three phase 3 trials.6 More than 2,800 patients have been studied. Sign endpoints included conjunctival hyperemia and corneal fluorescein staining, and the symptom endpoint of patient-reported ocular discomfort severity score was evaluated.
The trials met their primary endpoint of statistically significant change from baseline in ocular discomfort severity scores at day 15. Significant differences between the treatment and vehicle groups observed at day 3 were maintained throughout the remaining 11 days of treatment. In two of the phase 3 trials, statistically significant improvement in total corneal staining was observed after 2 weeks.
As an investigator in all four trials, I found it exciting to see the rapid improvement in corneal staining and to meet the trials’ conjunctival hyperemia endpoint. The incidence of IOP elevation (defined as an increase from baseline of > 5 mm Hg that resulted in IOP ≥ 21 mm Hg) was low and similar to vehicle. I hope to see further development and approval of KPI-121 0.25% so that I can implement its use for short-term treatment of DED in my patients.
1. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334-365.
2. Gupta PK, Drinkwater OJ, VanDusen KW, Brissette AR, Starr CE. Prevalence of ocular surface dysfunction in patients presenting for cataract surgery evaluation. J Cataract Refract Surg. 2018;44(9):1090-1096.
3. Starr CE, Gupta PK, Farid M, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45(5):669-684.
4. Schopf L, Enlow E, Popov A, Bourassa J, Chen H. Ocular pharmacokinetics of a novel loteprednol etabonate 0.4% ophthalmic formulation. Ophthalmol Ther. 2014;3(1-2):63-72.
5. Comstock TL, Sheppard JD. Loteprednol etabonate for inflammatory conditions of the anterior segment of the eye: twenty years of clinical experience with a retrometabolically designed corticosteroid. Expert Opin Pharmacother. 2018;19(4):337-353.
6. Holland EJ, Nichols KK, Foulks GN, Gupta PD, Sall K, Coultas SL. Efficacy and Safety of KPI-121 0.25% for Short Term Relief in Dry Eye (STRIDE). Electronic poster presented at: 2020 ASCRS Virtual Meeting; May 16-17, 2020.
OC-01 Nasal Spray: Novel Administration Route Avoids Ocular Side Effects
By Vance Thompson, MD, FACS

A dry eye treatment recently cleared a major development hurdle by meeting primary and secondary endpoints for both signs and symptoms in a phase 3 clinical trial. The ONSET-2 trial, conducted at 22 US centers and including 758 patients, found that OC-01 (Oyster Point Pharmaceuticals) improved Schirmer test scores and symptom endpoints in patients who received the treatment in comparison with controls.1
OC-01, a highly selective nicotinic acetylcholine receptor (nAChR) agonist, is formulated as a preservative-free nasal spray and employs a novel mechanism of action. The drug binds to cholinergic receptors on the trigeminal nerve, which is accessed via the anterior nasal cavity. This activates the trigeminal parasympathetic pathway responsible for innervating the entire lacrimal functional unit (Figure 1), activating natural tear production and restoring tear film homeostasis.
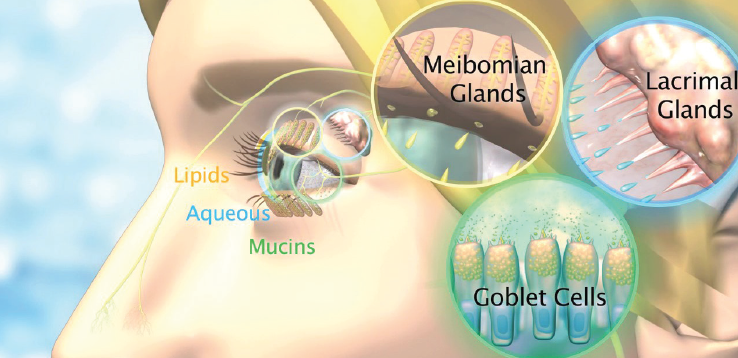
Figure 1. OC-01 increases the production of natural tears, with components from the entire lacrimal functional unit including the lacrimal glands (aqueous), meibomian glands (lipids), and goblet cells (mucins).
Courtesy of Oyster Point Pharmaceuticals
In the ONSET-2 trial, patients used one of two doses of the nasal spray (0.6 or 1.2 mg/mL) b.i.d. for 4 weeks or a control spray. The top-line data suggest that this drug has a rapid onset of action, exerting a positive effect on signs and symptoms in as little as 2 weeks. Both tested doses of OC-01 nasal spray met the primary endpoint of achieving a 10 mm or greater increase in Schirmer score in a statistically significantly greater percentage of patients than control. The mean gain in Schirmer score was approximately 11 mm in the treatment groups, compared to less than 6 mm in the control group. Additionally, patients treated with OC-01 experienced statistically significant gains in subjective eye dryness score compared to controls at 2 and 4 weeks.
The ONSET-2 trial enrolled patients across the spectrum from mild to severe dry eye, making the study population representative of the patients we see in clinical practice daily. This drug seems to work in a broad range of patients, with nearly 50% achieving improvements in clinical signs of DED.
Due to its unique route of administration as a nasal spray, there are no ocular side effects of OC-01. In the study, the most common adverse event was sneezing, transient in most patients. Adverse events were overwhelmingly mild in nature.
It is likely that Oyster Point will file a new drug application with the FDA this year, with a product launch expected in late 2021. I look forward to having this unique new drug available to bring relief to our patients with DED.
1. Oyster Point Pharma announces positive results in ONSET-2 phase 3 trial of OC-01 nasal spray for the treatment of the signs and symptoms of dry eye disease [press release]. Oyster Point Pharma. May 11, 2020. https://investors.oysterpointrx.com/news-releases/news-release-details/oyster-point-pharma-announces-positive-results-onset-2-phase-3. Accessed June 17, 2020.
OTX-CSI: A Sustained-Release Version of a Familiar Antiinflammatory Compound
By Eric D. Donnenfeld, MD

DED is one of the most common ophthalmic disorders. Historically, this condition was managed primarily with artificial tears and punctal occlusion. Over the past 2 decades, however, the management of DED has changed dramatically with the recognition that inflammation is a major contributor.
There are now three FDA-approved medications that address inflammation as a means to manage DED: cyclosporine ophthalmic emulsion 0.05% (Restasis, Allergan); cyclosporine ophthalmic solution 0.09% (Cequa, Sun Ophthalmics); and lifitegrast ophthalmic solution 5% (Xiidra, Novartis). These medications are immunomodulators targeting T cell function and are dosed twice a day, dramatically improving the management of DED.
As with all medications given as topical drops, however, contact time with the eye is limited, and there are dramatic peaks and troughs that affect the pharmacokinetics of drug delivery. A high concentration, given to create a peak, may result in burning and irritation—the most common medication-induced side effects— resulting in poor compliance and medication discontinuation.
INTRACANALICULAR INSERT FOR DED
An exciting drug delivery device in the pipeline has been designed to address some of the limitations of topical ophthalmic immunomodulator eye drops. OTX-CSI (Ocular Therapeutix) is a cyclosporine ophthalmic insert intended for intracanalicular use. It looks like, and inserts similarly to, the dexamethasone ophthalmic insert 0.4 mg (Dextenza, Ocular Therapeutix).
OTX-CSI combines the clinical benefits of cyclosporine with the advantages of punctal occlusion to provide two therapeutic approaches to DED with a single device. The insert contains 0.36 mg of cyclosporine and is designed to deliver therapeutic levels of drug onto the ocular surface along with punctal occlusion for a duration of approximately 12 weeks.
Continuous release of cyclosporine from this hydrogel insert may result in higher daily concentrations of cyclosporine delivered to the ocular surface than is achieved with topical cyclosporine, but with much lower peaks that should result in a low incidence of burning and stinging.
The two active formulations of OTX-CSI being studied in a phase 2 clinical trial (NCT04362670) contain the same cyclosporine dose and release drug at the same daily rates, but they differ in the composition of the hydrogel. Each hydrogel is designed to have a unique breakdown period, with one formulation intended to last approximately 2 to 3 months and the other approximately 3 to 4 months.
The objectives of the trial are to assess the safety, tolerability, and efficacy of OTX-CSI in individuals with DED. Additionally, two placebo groups will receive hydrogel inserts without cyclosporine that will degrade over different time frames. The phase 2 trial is now enrolling patients.
This is an exciting drug in the pipeline for the management of DED.
PKX-001: Moving Forward in Development for DED
An anti-aging glycopeptide (AAGP) molecule demonstrated a protective effect in preclinical testing in a mouse model of DED, according to developer ProtoKinetix.1 In April, the company announced these preliminary efficacy and safety results for its AAGP molecule PKX-001 in the treatment of DED.
Topical application of 5% PKX-001 q.i.d. in mice reduced desiccating stress–induced corneal permeability comparably to control vehicle (cyclosporine A microemulsion). Additional tests are ongoing. Previous animal model studies showed favorable in vitro and in vivo safety profiles for PKX-001 formulated in balanced saline solution at concentrations up to 10%. PKX-001 was well tolerated in treated eyes, was not considered an eye irritant, was negative in the bacterial reverse mutation assay, and did not induce chromosomal damage. It also reduced antiinflammatory cytokine levels, decreased oxidative stress, and increased cellular survival and improved functional activity under various stress conditions.
The company said it plans to move forward with the next stages of development.
1. https://eyewire.news/articles/protokinetix-aagp-exhibits-efficacy-and-safety-in-model-of-dry-eye-disease/. Accessed June 24, 2020.
Tarsus TP-03: Potential Game-Changer for Demodex Blepharitis
By Ehsan Sadri, MD, FACS, FAAO

Treating Demodex blepharitis is challenging. Lid scrubs with baby shampoo, warm compresses, and combination steroid-antibiotic ointments and oral treatments can help to alleviate symptoms, but none of these are toxic to Demodex folliculorum mites, which are implicated as the cause of blepharitis in 45% of cases.1 Persistent and chronic Demodex blepharitis can lead to a variety of conditions, including allergy, inflammation, lash loss and misdirection, telangiectasia, and decreased visual acuity.
Luckily, a new treatment is on the horizon that can help to kill Demodex. TP-03 (Tarsus Pharmaceuticals) is a topical drug that kills the mites by targeting their nervous system. To date, consistent results have been shown with TP-03 across four phase 2 studies and more than 100 patients. It is being developed in a multidose preserved formulation.
STUDY SUMMARY
In the single-arm, open-label Mars phase 2a study to evaluate the safety and efficacy of TP-03, 15 patients with Demodex blepharitis received one drop of the treatment in each eye b.i.d. for 28 days. Beginning at day 14 and continuing throughout the 90-day study period, TP-03 statistically significantly decreased the mean collarette score and mite density. Specifically, a 2-grade improvement on a four-point collarette score scale and a 10-fold improvement in mite eradication were demonstrated by the end of the 28-day treatment period.
No adverse events were reported, and there were no clinically significant changes in visual acuity, IOP, or slit-lamp biomicroscopy findings. Further, the use of TP-03 over the 4-week period was well-tolerated, with no patient discontinuing its use due to tolerability issues.
FUTURE OUTLOOK
TP-03, in my opinion, is going to be a game-changer. Patients with Demodex blepharitis are historically hard to treat because the condition is persistent and the therapies we currently have—mainly lid scrubs, warm compresses, and even combination steroid-antibiotics—do nothing to treat the underlying cause.
If we can kill the Demodex folliculorum mites, we can help our patients to achieve increased comfort and to maximize their visual acuity. This is important to all patients, but especially to our premium IOL patients who have high expectations for their visual outcomes. A pharmaceutical agent that can rapidly, completely, and effectively treat Demodex blepharitis and that is well-tolerated and safe is severely needed. TP-03 is a promising new agent to fulfill that need.
Results from the phase 2b randomized controlled Jupiter study will be forthcoming.
1. Zhao YA, Wu LP, Hu L, Xu JR. Association of blepharitis with Demodex: a meta-analysis. Ophthalmic Epidemiol. 2012;19(2):95-102.
TTHX-1114: Engineered Growth Factor With Potential for Treatment of Endothelial Deficiency
By William B. Trattler, MD

The current standard of care for Fuchs and corneal endothelial dystrophies focuses on increasing the number and function of corneal endothelial cells via transplantation of donor tissue. In 2019, more than 35,000 endothelial transplant procedures were performed in the United States, and that number is on the rise.1 Corneal transplantation can effectively restore vision; however, postoperative recovery is challenging, and most patients undergo long-term chronic steroid therapy to minimize the risk of graft rejection.2,3
An alternative approach to the management of endothelial dystrophy is pharmacologic stimulation of the endogenous corneal endothelial cells to promote proliferation, migration, and regeneration. A novel drug with an engineered fibroblast growth factor (FGF) holds promise to help restore healthy endothelial function. Clinical trials are expected to begin this summer.
TTHX-1114 (Trefoil Therapeutics) is closely related to the molecule FGF-1, one of 22 naturally occurring FGFs. FGF-1 is the only FGF molecule that targets all seven FGF receptor isoforms; however, it has a short biological half-life due to its susceptibility to proteolysis and cysteine oxidation, making it unsuitable as a drug.
Trefoil Therapeutics has stabilized FGF-1 by substituting key amino acids to decrease the flexibility of the protein. This new engineered formulation does not introduce any new functionalities of the protein on a qualitative basis. TTHX-1114 contains three stabilizing mutations, one of which is the introduction of an internal disulfide bond. The compound is effective at much lower applied concentrations than FGF-1, but qualitatively its biological activity is identical. TTHX-1114 is expected to have the same mechanism driven effects as FGF-1 with no unexpected activity.
It is expected that treatment with TTHX-1114 will require four to six intracameral injections administered over a period of 6 to 12 months. At that point, we anticipate that patients’ endothelial cell counts will recover, and healthy endothelial function will be restored.
CONCLUSION
Patients with Fuchs dystrophy who experience vision loss often defer treatment because it involves surgery. Having a nonsurgical option such as treatment with TTHX-1114 could lead to earlier intervention and better visual outcomes for patients.
1. Eye Bank Association of America. 2019 Statistical Report. https://eyelearn.restoresight.org/resources. Accessed June 25, 2020.
2. Allan BD, Terry MA, Price FW Jr, Price MO, Griffin NB, Claesson M. Corneal transplant rejection rate and severity after endothelial keratoplasty. Cornea. 2007;26(9):1039-1042.
3. Price MO, Scanameo A, Feng MT, Price FW Jr. Descemet’s membrane endothelial keratoplasty: risk of immunologic rejection episodes after discontinuing topical corticosteroids. Ophthalmology. 2016;123(6):1232-1236.




