
In the end, it is best to pick the procedure you are most comfortable with.
By Penny A. Asbell, MD, FACS, MBA, FARVO
Perhaps the best predictor of significant dry eye disease (DED) after refractive surgery is the presence of DED before surgery. Therefore, the key to good decision-making in planning refractive surgery is to determine the severity of DED before you select and perform any procedure.
TAKE THE PROPER STEPS TO DETECT, TREAT DED
There is no pathognomonic test for DED. The key to identifying DED, especially mild DED, starts with the patient dialogue. Asking patients about their ocular discomfort can help you determine the presence and level of DED. One way to get patients to share this information is to ask them to complete a DED questionnaire prior to their eye examination. Popular questionnaires include the Standardized Patient Evaluation of Eye Dryness (SPEED), the Ocular Surface Disease Index (OSDI), and the National Eye Institute Visual Function Questionnaire-25. Experimenting with these and other questionnaires will help you determine which works best for your practice.
When patients report some level of ocular discomfort, consider a topical anesthetic. First evaluate the eye without the anesthetic and then after its placement. If the discomfort (eg, dryness, irritation) disappears, then it is quite likely that ocular surface disease is present; this should be addressed prior to scheduling patients for any ocular procedure, especially refractive surgery. If the discomfort persists at the same level despite administration of a topical anesthetic, then the source of pain is uncertain, possibly indicating that the patient is at increased risk for postoperative chronic pain. Significant neuropathic pain after refractive surgery is rare, but, when it occurs, these patients are often the most unhappy. This is a situation we would like to avoid creating by performing refractive surgery.
Acquiring informed consent before surgery is crucial to ensure that patients understand the risks and benefits of refractive surgery. Unknowns include the presence and severity of DED postoperatively.

WHICH PROCEDURE IS BEST?
Evidence-based data on the risk of DED after refractive surgery procedures have yielded little randomized comparative data to date, but one meta-analysis suggested that small-incision lenticule extraction (SMILE) is less likely to induce postoperative dryness than LASIK.1 PRK is likely the most painful in the immediate postoperative period, as both superficial and subbasal nerves are ablated.2 Evaluation of corneal nerves months after refractive surgery showed that the nerves returned to their preoperative density by 2 to 3 years after PRK,3 but it took up to 5 years after LASIK.4 Also, nerve density may be reduced as much as 1 decade after LASIK.5
These data suggest that postoperative DED and ocular pain influence not only what happens in the short term after refractive surgery but also what changes persist over time, what type of healing occurs, and to what extent healing, especially of the corneal nerves, occurs.
Most refractive surgery patients are happy with their results, and even those who have some element of dryness typically feel that the benefits outweigh the risks. Long-term effects of refractive surgery on DED status seem to be less predictable, but the risk of significant postoperative DED is evident, even in patients with no or mild preexisting DED.
In patients with minimal signs and symptoms of mild DED and ocular surface disease preoperatively, it is likely that any refractive surgery procedure can be safe and effective. For these patients, the surgeon should pick the procedure that he or she is most comfortable with and the one that is most likely to accurately correct the refractive error.
1. Kobashi H, Kamiya K, Shimizu K. Dry eye after small incision lenticule extraction and femtosecond laser-assisted LASIK: meta-analysis. Cornea. 2017;36(1):85-91.
2. Rabina G, Boguslavsky II, Mimouni M, Kaiserman I. The association between preoperative dry eye symptoms and postoperative discomfort in patients underwent photorefractive keratectomy. J Ophthalmol. 2019;2019:7029858.
3. Srinivasan S. Feeling better and healing faster. J Cataract Refract Surg. 2019;45:897-898.
4. Xia L, Zhang J, Wu J, Yu K. Comparison of corneal biological healing after femtosecond LASIK and small incision lenticule extraction procedure. Curr Eye Res. 2016;41(9):1202-1208.
5. Cetinkaya S, Gulmez M, Mestan E, Ucar F, Ali N. Influence of incision size on dry eye symptoms in the small incision lenticule extraction procedure. Cornea. 2019;38(1):18-23.


Once the condition is controlled, the choice is yours.
By Kristen Ashourian, MS; and William B. Trattler, MD
Patients interested in reducing their need for contact lenses or glasses often have underlying DED. It is therefore important to evaluate patients for DED during preoperative examinations for refractive surgery, whether PRK, LASIK, SMILE, phakic IOL implantation, corneal inlay implantation, or refractive lens exchange.
Although much of the focus over the past 2 decades has been on aqueous-deficiency DED, recently greater attention has been placed on meibomian gland dysfunction (MGD) and blepharitis, along with tear quality. For this reason, the preoperative examination should include evaluation of not only the ocular surface but also the lids and lashes in order to screen for MGD and blepharitis.
The good news is that DED, MGD, and blepharitis can be treated in advance of refractive surgery, allowing many patients with these conditions to be appropriate candidates for surgery.
IDENTIFY AND TREAT DED
The preoperative examination allows the surgical team to determine whether a patient is an appropriate candidate for refractive surgery and to decide which procedure will provide the best outcome for that patient. DED has the potential to affect preoperative measurements, including UCVA and BCVA, refraction, keratometry, topography, and wavefront assessments. Patients with DED can present with loss of BCVA and topographic abnormalities (Figure 1).
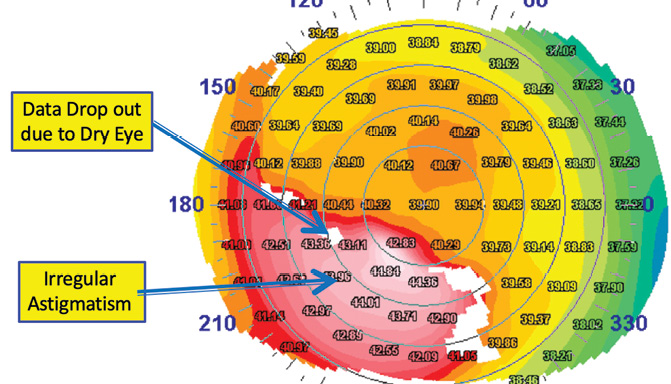
Figure 1. Topographic abnormalities are present in this eye. Figures 1 and 2 courtesy of William B. Trattler, MD
Patients identified with DED and/or MGD can be treated for their conditions before a final preoperative examination to determine if the condition has improved. Patients may experience significant improvement in BCVA and can demonstrate different refractive errors compared with the baseline measurement.
Topography and wavefront aberrometry may also become more regular with treatment of DED. It is important to note, however, that in some instances of DED with irregular corneal topography, treatment of the DED may not lead to improvement in the uniformity of the topography. In these cases, clinicians should look carefully for underlying corneal conditions, including epithelial basement membrane dystrophy and early keratoconus.
TESTING, TESTING
During the preoperative examination, an assessment of the health of the ocular surface is performed, along with an evaluation of the lids and lashes. One of the key tests is placement of fluorescein in the tear film. Patients with DED or with MGD-associated blepharitis will have a rapid tear breakup time (< 7 seconds) and will often exhibit punctate epithelial erosion on the cornea (Figure 2).
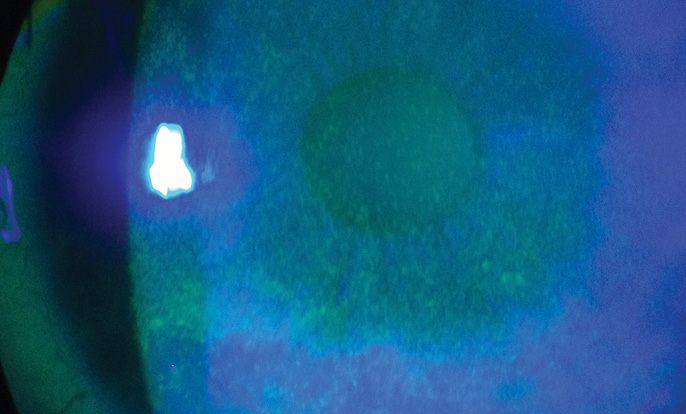
Figure 2. Punctate epithelial erosion on the cornea.
During this part of the examination, evaluation of the cornea can also reveal subtle forms of epithelial basement membrane dystrophy or Salzmann nodular degeneration. Patients with a history of long-term contact lens wear may have areas of staining superiorly that have been termed advancing wave-like epitheliopathy.1 Assessment of DED and MGD during the preoperative examination can also include Schirmer testing, tear film osmolarity testing, evaluation of tear film inflammation, and the use of lissamine green dye.
Assessment should also include an evaluation of the meibomian glands. Although many patients with MGD or blepharitis will have obvious clinical signs at the slit lamp, some may have nonobvious MGD. Pressure can be placed on the eyelids to assess the quality of meibomian gland secretions. Imaging of the meibomian glands with the LipiView II Ocular Surface Interferometer (Johnson & Johnson Vision) or other technologies can provide a better understanding of the health of the glands.
Diagnostic tools to determine the health of the tear film include topography, which can show telltale findings such as image dropout. Also, with repeated wavefront measurements, significant differences from one measurement to the next are signs of an inadequate tear film. Some refractive surgeons also use the HD Analyzer (Visiometrics) in assessments of DED.
TREATMENT OPTIONS
When DED or MGD and blepharitis are identified, it is important to treat these conditions and then have patients return for repeat measurements.
Preoperative treatments for DED and MGD include many options. Topical cyclosporine ophthalmic emulsion 0.05% (Restasis, Allergan) and lifitegrast ophthalmic solution 5% (Xiidra, Shire), along with topical steroids and/or artificial tears, are first-line therapies for aqueous-deficiency DED. Warm compresses, topical steroids, hypochlorous acid spray, and topical azithromycin are first-line therapies for MGD and blepharitis.
Recently, several in-office procedures for MGD and blepharitis, including BlephEx (Scope Ophthalmics), LipiFlow Thermal Pulsation System (Johnson & Johnson Vision), TearCare (Sight Sciences), iLux (Tear Film Innovations), intense pulsed light (multiple vendors), and MiBo Thermoflo (Mibo Medical Group), have moved into a first-line position in many clinical practices.
Regardless of which technologies are used for treatment of MGD and blepharitis, the important step is to bring patients back for repeat measurements after treatment to determine if the measurements have improved so that a final refractive surgical plan can be developed.
When DED and MGD are treated to a level at which patients have mild symptoms or less, patients can typically proceed to refractive surgery. Because DED, MGD, and blepharitis are chronic conditions, it is not possible to permanently eliminate these conditions.

SURGERY CHOICE
Choice of refractive surgical procedure is not affected by a history of DED or MGD and blepharitis. Any patient can have a temporary worsening of DED after any refractive surgical procedure, whether LASIK, PRK, SMILE, phakic IOL, corneal inlay, or refractive lens exchange.
One challenge is that, even without refractive surgery, patients on therapy for DED or MGD can return throughout the year with worsening of their conditions. With this in mind, it is important to continue to evaluate and treat patients for them after refractive surgery.
CONCLUSION
Refractive surgery can provide significant benefits to patients. DED, MGD, and blepharitis can be identified and treated before refractive surgery to help improve refractive outcomes. Because these conditions are lifelong, continued evaluation and treatment of DED and MGD will be required after refractive surgery.
Although no cure has been developed for either DED or MGD, many therapies are available that can significantly help patients with these conditions. In the vast majority of cases, therefore, patients can safely and effectively undergo elective refractive surgery and can expect to experience significant improvements in their vision.
1. D’Aversa G, Luchs JL, Fox MJ, Rosenbaum PS, Udell IJ. Advancing wave-like epitheliopathy. Clinical features and treatment. Ophthalmology. 1997;104(6):962-969.


The optimum option is to treat preexisting disease preoperatively.
By Alice T. Epitropoulos, MD, FACS; and Curtin G. Kelley, MD
Dry eye is the most common, and potentially the most devastating, nonkeratome-related complication associated with refractive surgery. DED can have a substantial impact on a patient’s quality of vision after any type of refractive surgical procedure. Symptoms can range from mild ocular irritation to severe discomfort and vision loss.
What is the best refractive surgical procedure for a patient with mild DED? We would argue that the best procedure is prevention: that is, detecting DED, educating the patient about it, and treating it before proceeding with surgery. If these precautions are taken, the risk of the patient’s experiencing worsened DED after surgery is minimized, and the potential for a happy patient is maximized.
DETECTING DED
One of the most important ways to prevent postoperative DED is to carefully screen and identify patients at risk for DED and to optimize the health of their tear film before refractive surgery. Refractive surgeons should take steps at every stage of the surgical process to maximize results and minimize postoperative DED.
There is often a mismatch between severity of signs and symptoms in patients with DED; in fact, a high percentage of patients who seek refractive surgery do not even realize they have DED. Any mention by the patient of contact lens intolerance should strongly suggest the possibility of underlying DED.
Staining of the conjunctiva or cornea is a sign of tissue damage from DED that can cause fluctuations in vision and inconsistent refractions and can ultimately affect postoperative visual acuity and other outcomes. Lissamine green or rose bengal dyes can be used to evaluate conjunctival staining; fluorescein should be used to evaluate corneal staining and the stability of the tear film. Corneal staining can be particularly significant if it involves the visual axis. Patients who have conjunctival or central corneal staining should not be candidates for refractive surgery until the ocular surface has been treated appropriately (Figure 3).
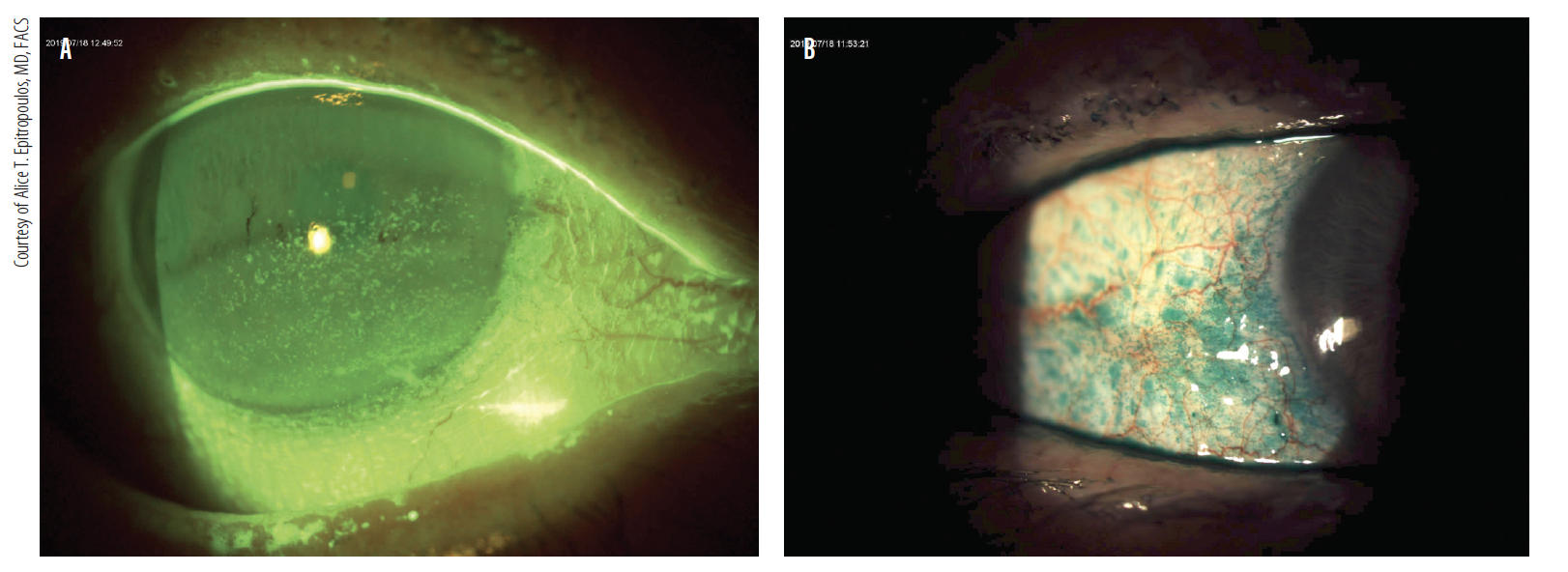
Figure 3. Patients who have keratitis and conjunctival or central corneal staining (A,B) should not be candidates for refractive surgery until the ocular surface has been treated appropriately. Courtesy of Alice T. Epitropoulos, MD, FACS
Another red flag is a complaint of fluctuation in vision. Patients with this symptom are likely to have a component of evaporative DED, and surgery should not proceed until the surface is rehabilitated.
These and other ocular surface problems can be relative—and sometimes absolute—contraindications for refractive surgery. However, with effective management, the incidence and severity of DED related to refractive surgery can be minimized.
WHICH PROCEDURE?
Any type of laser vision correction procedure, including LASIK, PRK, and SMILE with the VisuMax femtosecond laser (Carl Zeiss Meditec), can alter the ocular surface environment and induce tear film instability. In fact, most patients experience at least transiently dry eyes after laser vision correction. There has been debate regarding which refractive procedure or procedures carry the least amount of risk of exacerbating preexisting DED.
PRK and LASIK have become established safe and effective procedures that result in excellent visual outcomes when used according to their labeling in appropriately selected candidates. LASIK is the most popular refractive procedure performed in the United States. Advantages include fast, painless visual rehabilitation and less regression and corneal haze than are seen with PRK. Surface procedures such as PRK, however, remain excellent options for mild to moderate refractive corrections, particularly for patients with thin corneas, recurrent erosions, or a predisposition to trauma. Further, there is no risk of flap-related complications with PRK.
Patient satisfaction with LASIK is high, but DED is a common postoperative complication. The prevalence of DED symptoms in patients before undergoing LASIK has been estimated to be between 38% and 75%.1,2 Some patients experience transient DED, and others report more severe symptoms over the long term.
When evaluating a patient with a history of DED, we first consider performing a surface procedure. It is widely thought that the creation of the LASIK flap severs corneal nerves, resulting in decreased corneal sensation, whereas surface ablation leaves the corneal nerves intact.2 On the other hand, Schallhorn has documented a slight increase in symptoms of DED at 3 months after PRK.3
If LASIK is chosen instead of PRK, we aim to use a broad hinge because DED symptoms have been shown to be greater in eyes with narrow hinges.2,4,5 A flap with a wide hinge (range, 6–7.5 mm) severs a smaller number of corneal nerves than a flap with a narrow hinge (range, 3–5.5 mm).4 Placing a LASIK hinge nasally rather than superiorly may also result in fewer symptoms of DED.2,6,7 The long posterior corneal nerves enter the eye nasally and temporally. A superior hinge will transect both of these arms, whereas a nasal hinge will transect only the temporal arm. Additionally, thin flaps (approximately 110 μm) have been associated with less risk of a neurotrophic cornea and subsequent DED.8
The use of a femtosecond laser for LASIK flap creation (FS-LASIK) generates more consistent and predictable flap thickness and diameter and hinge width than traditional microkeratome flap creation, and this may reduce the risk of flap-associated complications such as corneal nerve injury and subsequent DED.5,9,10
Some published data suggest that, although neither procedure causes more dryness than the other, both PRK and LASIK cause some dryness. Marakami and Manchee found that symptoms of DED, including visual fluctuations and foreign body sensation, returned to baseline in both LASIK and PRK patients after 1 year.11
In a SMILE treatment, a femtosecond laser shapes a refractive lenticule that is removed mechanically through an arcuate sidecut. Potential benefits of SMILE include reduction of complications associated with the cutting of the flap, greater corneal tectonic strength, and less impact on corneal nerves.12-16 Such benefits may also reduce the exacerbation of DED.
Recent studies have suggested that there is less risk of aggravating preexisting DED with SMILE than with myopic FS-LASIK. A recent meta-analysis suggested that patients experienced milder subjective DED symptoms with SMILE. The procedure did not demonstrate superiority over FS-LASIK, however, and both procedures were comparable in terms of efficacy, predictability, and safety.14
CONCLUSION
DED is extremely common after refractive surgery, even in patients with no history of the condition. In fact, DED is the most common reason for patient dissatisfaction after LASIK. Symptoms are common, particularly in the first few months after surgery, and tend to subside with time.
Patients should be screened and examined carefully before any proposed refractive procedure for signs of ocular surface disease and treated to optimize the health of the ocular surface. This will help to maximize both the accuracy of the procedure—whatever refractive surgery procedure is chosen—and the comfort of the patient postoperatively.
Patients with DED who are considering keratorefractive surgery, particularly LASIK, should be cautioned that, even if they are asymptomatic preoperatively, this preexisting condition could become worse after surgery. Refractive surgeons should review the advantages and disadvantages of each refractive surgical procedure with potential patients, and they should always keep in mind the necessity of careful and appropriate patient selection.
In the end, patients with DED can safely undergo refractive surgery if they are counseled and if their preexisting condition can be improved preoperatively.17 No matter which refractive surgical procedure is chosen, the best way to prevent postoperative DED is to identify it and treat it preoperatively.
1. McGhee CN, Orr D, Kidd B, et al. Psychological aspects of excimer laser surgery for myopia: reasons for seeking treatment and patient satisfaction. Br J Ophthalmol. 1996;80(10):874-879.
2. De Paiva C, Chen Z, Koch D, et al. The incidence and risk factors for developing dry eye after myopic LASIK. Am J Ophthalmol. 2006;141(3):438-445.
3. Schallhorn J. Challenging conventional wisdom about LASIK. Paper presented at: American Society of Cataract and Refractive Surgery Annual Meeting; May 3-7, 2019; San Diego, CA.
4. Donnenfeld ED, Ehrenhaus M, Solomon R, et al. Effect of hinge width on corneal sensation and dry eye after laser in situ keratomileusis. J Cataract Refract Surg. 2004;30:790-797.
5. Friedlaender MH. LASIK surgery using the IntraLase femtosecond laser. Int Ophthalmol Clin. 2006;46:145-153.
6. Donnenfeld ED, Solomon K, Perry HD, Doshi SJ. The effect of hinge position on corneal sensation and dry eye following LASIK. Ophthalmology. 2003;110(3):1023-1029.
7. Feng YF, Yu JG, Wang DD, et al. The effect of hinge location on corneal sensation and dry eye after LASIK: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2013;251(1):357-366.
8. Barequet IS, Hirsh A, Levinger S. Effect of thin femtosecond LASIK flaps on corneal sensitivity and tear function. J Refract Surg. 2008;24(9):897-902.
9. Binder PS. Flap dimensions created with the IntraLase FS laser. J Cataract Refract Surg. 2004;30:26-32.
10. Kezirian GM, Stonecipher KG. Comparison of the IntraLase femtosecond laser and mechanical keratomes for laser in situ keratomileusis. J Cataract Refract Surg. 2004;30:804-811.
11. Marakami Y, Manchee EE. Prospective, randomized comparison of self-reported postoperative dry eye and visual fluctuation in LASIK and photorefractive keratectomy. Ophthalmology. 2012;119(11):2220-2224.
12. Ivarsen A, Asp S, Hjortdal J. Safety and complications of more than 1500 small-incision lenticule extraction procedures. Ophthalmology. 2014;121(4):822-828.
13. Zhang Y, Shen Q, Jia Y, Zhou D, Zhou J. Clinical outcomes of SMILE and FS-LASIK used to treat myopia: a meta-analysis. J Refract Surg. 2016;32(4):256-265.
14. Shen Z, Shi K, Yu Y, et al. Small incision lenticule extraction (SMILE) versus femtosecond laser-assisted in situ keratomileusis (FS-LASIK) for myopia: a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158176.
15. Kobashi H, Kamiya K, Shimizu K. Dry eye after small incision lenticule extraction and femtosecond laser-assisted LASIK: meta-analysis. Cornea. 2017;36(1):85-91.
16. Pietilä J, Huhtala A, Makinen P. Uncorrected visual acuity, postoperative astigmatism, and dry eye symptoms are major determinants of patient satisfaction: a comparative, real-life study of femtosecond laser in situ keratomileusis and small incision lenticule extraction for myopia. Clin Ophthalmol. 2018;12:1741-1755.
17. American Academy of Ophthalmology. Dry Eye Syndrome PPP – Nov 2018; AAO PPP Cornea/External Disease Committee, Hoskins Center of Quality Eye Care.




