IVMED-80, a twice-daily eye drop for the treatment of keratoconus in development by iVeena Delivery Systems, was recently granted orphan drug designation by the FDA. This drug, with its copper-based formulation, is reportedly the first eye drop designed to treat keratoconus without the need for adjunctive laser treatment or surgical intervention. Human clinical trials are set to begin in the first half of this year.
Early animal studies have shown that the eye drop induced central flattening and increased corneal stiffening in vivo in rabbits (Figure 1),1 and human cadaver studies have shown that the formulation increased lysyl oxidase (LOX) activity and corneal stiffness in human keratoconic corneas ex vivo.2 Low LOX activity in the cornea has been linked to the development of keratoconus both genetically and biochemically.3 The IVMED-80 formulation is based on a cofactor for LOX. Detailed results from these early studies will be reported at ARVO in May.
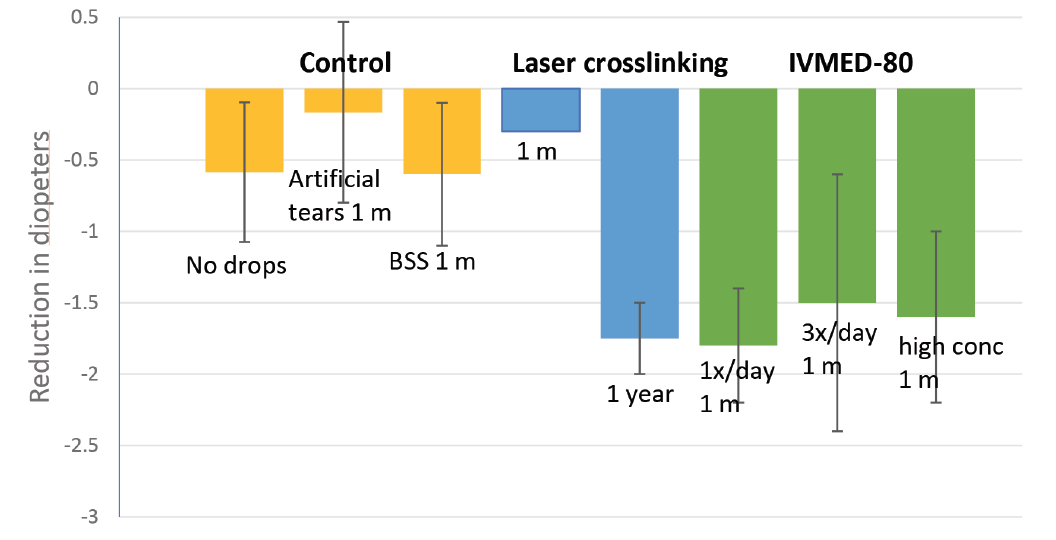
Figure 1. IVMED-80 was effective in decreasing corneal diopter measurements in rabbits compared with control treatments. The eye drop achieved improvement in corneal elastic modulus within 1 to 4 weeks of therapy to a level comparable to conventional CXL at 1 year after laser surgery in rabbits.
EARLY STUDY OUTCOMES
The early in vivo and ex vivo studies were led by principal investigator Sarah Molokhia, PhD, iVeena’s director of research and development. In an interview with CRST, Dr. Molokhia said that, in both the in vivo animal studies and the human cadaver ex vivo studies, IVMED-80 showed an effect by 6 weeks after drops were administered. “With the animals, we looked at topography as well as OCT images, and we have seen corneal flattening and an increase in corneal biomechanical strength. And with the ex vivo keratoconic human corneas, we looked at the LOX activity of corneal fibroblasts as well as the corneal biomechanics—the stress-strain curves of the cornea—and so far we’ve seen an effect at 6 weeks or earlier in both models,” she said. “We are currently working with dosage and gaining a better understanding of the concentration and regimen. We plan on starting a phase 1-2a study, mainly for safety, within the coming 3 to 4 months.”
CLINICAL TRIAL AIMS
The aim of the upcoming clinical trial is to ascertain whether the treatment will require use throughout the patient’s life or if there is a dosage that can be taken over a period of time to provide long-term or permanent improvement in collagen stiffness. “I’m really interested in seeing if the effect will last; I want to understand how long patients will have to take the drops. The efficacy comes on quite quickly, but the question is: If the eye drop is stopped, will the effect end?” Dr. Molokhia said. “One way we are addressing this question is by designing the clinical trial with an arm that provides treatment for 6 weeks, and another that goes longer,” she explained.
The human clinical trials will target treatment in patients with mild to moderate keratoconus, and trial dosing will be twice daily, according to Michael Burr, MS, MBA, iVeena vice president of product development. “We think it will have applications in multiple phases of the disease progression,” he said.
INSPIRATION AND INNOVATION
IVMED-80 is the brainchild of cornea specialist and iVeena founder Bala Ambati, MD. Dr. Ambati, who is a professor of ophthalmology at the Moran Eye Center at the University of Utah, followed the CXL clinical trials with great interest and began to wonder if there might be a way to formulate an eye drop that could have the same effect as crosslinking. If that were possible, he reasoned, the result would be a noninvasive, relatively cost-effective topical therapy for the treatment of keratoconus. Dr. Ambati said, “Pharmacologic crosslinking without laser or surgery would be a game-changer for physicians and patients with a chronic disease currently treated with a surgery—epi-off [CXL]—that is very expensive, time-consuming, and has significant risks.”
Experimental Eye Drops: A Possible Step Forward in Keratoconus Management
A conservative, noninvasive treatment could increase corneal biochemical and biomechanical resistance against ectasia progression.
By Cosimo Mazzotta, MD, PhD

The FDA recently granted orphan drug status to IVMED-80 (iVeena), an eye drop for the treatment of keratoconus independent of surgery or laser treatment. This novel technology is based on a cofactor for lysyl oxidase (LOX) activity. According to preliminary data, low LOX activity in the cornea has been linked to the biochemical pathogenetic factors involved in the development of keratoconus.1-3
Lysyl oxidases, a family comprising LOX and four LOX-like enzymes (LOXL1-4), catalyze the formation of collagen crosslinks and elastin and are implicated in the age-related corneal stiffening synonymous with keratoconus. Although the pathogenesis of keratoconus is complex and unclear, one current hypothesis is based on alterations in the organization and structure of collagen fibrils and extracellular matrix.
According to the literature, all four LOX-like enzymes are present in each corneal layer as well as in the limbus and conjunctiva. A lower staining intensity of LOXL2 was found using immunohistochemistry and Western blot analysis in keratoconus specimens.1
THE CONNECTION
A meta-analysis evaluating the association of genetic variants in LOX with keratoconus demonstrated that two LOX variants (rs2956540 and rs10519694) may affect individual susceptibility to keratoconus; however, the results remained inconclusive because of the great heterogeneity existing across populations.2 Indeed, larger-scale and multiethnic genetic studies on keratoconus are required to further validate these results.
Recent studies evaluating the correlation of visual and keratometry outcomes after CXL in patients with keratoconus and cone epithelium-specific gene expression levels demonstrated that preoperative levels of molecular factors such as LOX aid in understanding CXL outcomes at the tissue level.3 The study elucidated the differential expression of a set of local molecular factors in the ectatic cone area of the cornea to uncover a functional cause for the focal corneal weakening characteristic of keratoconus in human corneal samples. Epithelial cells were collected from keratoconus patients (n = 66) undergoing CXL procedures from cone apex and periphery. Nonectatic refractive surgery patients (n = 23) served as controls.
The ratio of epithelial gene expression in the cone and periphery of each eye was estimated by quantitative polymerase chain reaction analysis and correlated with clinical data. Epithelium from the cone apex of keratoconus patients had elevated levels of the inflammatory factors tumor necrosis factor-α, interlukin-6, and matrix metalloproteinase 9 (MMP-9), but reduced LOX. This study provides the first evidence that altered corneal epithelial and stromal expression of specific genes at the corneal cone apex drives focal structural weakness in keratoconus.
EXPERIMENTAL EYE DROPS FOR KERATOCONUS
The experimental results of the iVeena eye drops’ effect on human keratoconic corneas showed an increase of LOX activity and corneal stiffening. According to morphologic evidence, an induced central corneal flattening in rabbits was demonstrated on OCT corneal scans.
In my opinion, this drug could represent a step forward for conservative noninvasive keratoconus management, enabling a relatively cost-effective treatment for keratoconus progression to be used as a first-line approach for the time necessary to gain a spontaneous stability of the ectatic disease (35–40 years) or in combination with CXL. If not sufficient alone, the IVMED-80 biochemical treatment could be combined with both epithelium-off and epithelium-on CXL. Particularly if clinical results demonstrate safety and efficacy, this therapy could be a noninvasive method to treat progressive keratoconus or an adjuvant treatment of epithelium-on CXL, improving its efficacy and reducing the side effects of epithelium removal such as corneal infections, postoperative pain, and wound-related complications.
Point-of-care tests are needed to detect specific concentration of LOX levels in order to predict keratoconus progression and response to combined therapies.
1. Dudakova L Sasaki T, Liskova P, Palos M, Jirsova K. The presence of lysyl oxidase-like enzymes in human control and keratoconic corneas. Histol Histopathol. 2016; 31(1):63-71.
2. Zhang J, Zhang L, Hong J et al. Association of common variants in LOX with keratoconus: A meta-analysis. PLoS One. 2015;10(12):e0145815.
3. Pahuja N, Kumar NR, Shroff R, et al. Differential molecular expression of extracellular matrix and inflammatory genes at the corneal cone apex drives focal weakening in keratoconus. Invest Ophthalmol Vis Sci. 2016;57(13):5372-5382.
The FDA’s Orphan Drug Act provides a bundle of benefits intended to spur the development of treatments for rare diseases. These benefits may include tax credits to offset clinical trial expenses and market exclusivity after approval.
Gerald Simmons, iVeena’s CEO, noted that the FDA’s orphan drug designation is quite significant. “What that essentially means is that we should be able to take the product through to commercialization, and, if it gets approved by the FDA, it will mean that we have the exclusive right to market an eye drop for keratoconus for 7 years. No other company would be able to market a similar topical eye drop for keratoconus throughout that time period. That exclusivity is extremely valuable because it eliminates the potential for competition in that timeframe, and that is invaluable, especially for a small emerging company like ours.” He says it is also powerful for a prospective corporate licensee because, “if we were to license this product to a large pharmaceutical company, it would secure the same exclusivity.”
Mr. Burr suggested that the orphan designation also has unwritten benefits. “What the orphan drug designation does for our company is it immediately gives us credibility because it represents a validation signal from the FDA that indeed we have developed something that has tremendous potential.”
1. Data on file; iVeena.
2. Data on file; iVeena.
3. Bykhovskaya Y, Li X, Epifantseva I, et al. Variation in the lysyl oxidase (LOX) gene is associated with keratoconus in family-based and case-control studies. Invest Ophthalmol Vis Sci. 2012;53(7):4152-4157.




