CASE PRESENTATION
A 47-year-old black woman with a remote history of LASIK and steroid-responsive advanced glaucoma in both eyes was referred by the Duke Glaucoma Service to the Cornea Service because of corneal decompensation involving her right eye.1
The patient’s extensive history included trabeculectomy in both eyes and the implantation of a »Baerveldt 350-mm2 implant (Johnson & Johnson Vision) in her right eye, with a subsequent exchange for a »Baerveldt 250-mm2 implant owing to the formation of a large bleb. Upon presentation to the Cornea Service, the visual acuity in her right eye measured 20/400, with a visual potential of 20/40 based on her examination prior to corneal decompensation.
The patient underwent a triple procedure combining cataract removal, IOL implantation, and Descemet stripping automated endothelial keratoplasty (DSAEK). The postoperative course was complicated by primary graft failure. A second graft was placed, but it failed shortly after additional glaucoma surgery consisting of tube removal and laser endocyclophotocoagulation.
A third DSAEK procedure was performed. On postoperative day 1, the graft was fully attached, but a small cleft of fluid was observed in the LASIK flap interface (Figure 1). Visual acuity measured 20/400, and the IOP measured 32 mm Hg, suggesting a mechanism similar to interface fluid syndrome (IFS) after LASIK.2,3
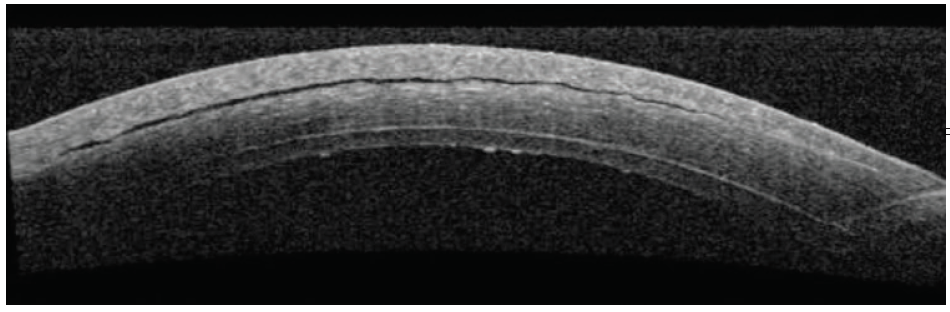
Figure 1. Initial anterior segment OCT (AS-OCT) shows a small gap in the LASIK flap.
The patient was unable to tolerate topical glaucoma medications, and the cleft of fluid worsened over the subsequent week (Figure 2). She was taken back to the OR. The surgeon attempted to drain the fluid through venting incisions while adding air in a rebubbling procedure, releasing fluid and collapsing the interface. Acetazolamide was administered intravenously during the procedure, and the patient was sent home with oral acetazolamide 500 mg.
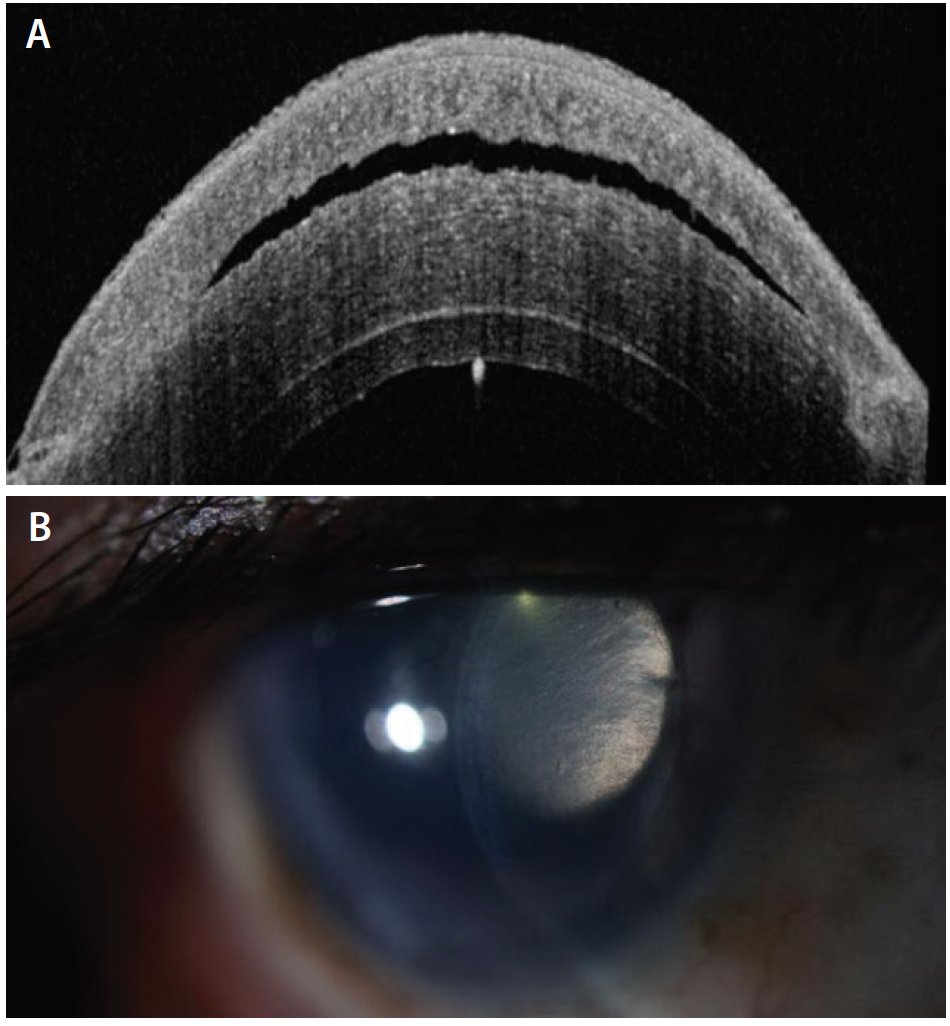
Figure 2. AS-OCT 10 days after the image shown in Figure 1 demonstrates increased fluid in the LASIK flap (A). Slit-lamp photograph taken at the same visit also shows a gap in the LASIK flap (B).
A week later, visual acuity in the patient’s right eye remained poor—count fingers at face—and the IOP measured 19 mm Hg. Slit-lamp photographs were taken at this time (Figure 3). Topical glaucoma therapy was restarted in an attempt to further lower the IOP. Over the subsequent weeks, the patient discontinued all topical glaucoma drops on her own because of ocular irritation. Her examinations have been relatively stable, with a poor visual acuity (currently 200E at 4 feet), an IOP in the low 20s mm Hg on oral acetazolamide, and a persistent fluid cleft.
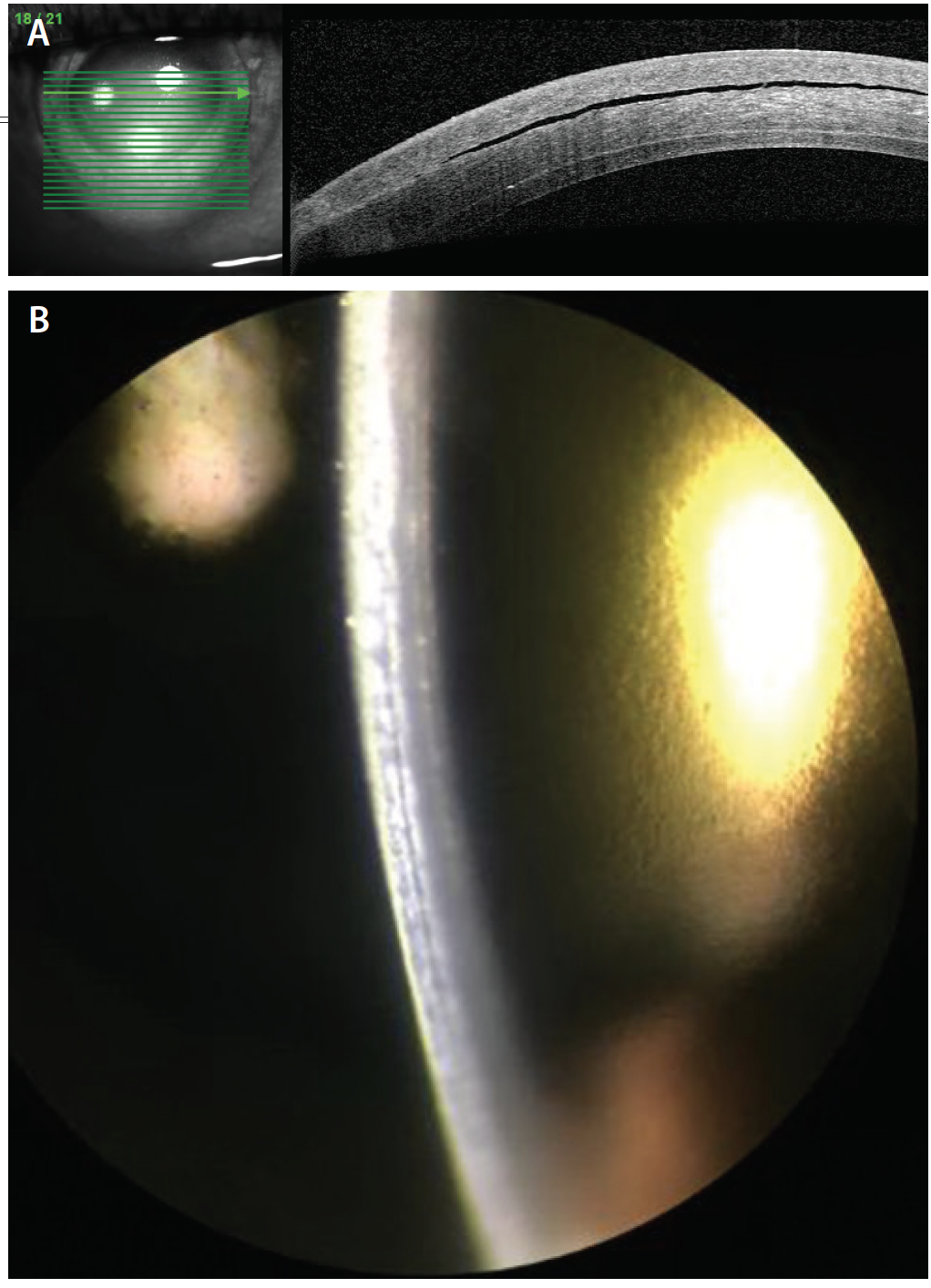
Figure 3. After a rebubbling procedure with venting incisions, AS-OCT shows a persistent— but smaller—cleft in the LASIK flap (A), and slit-lamp photography reveals a subtle gap in the LASIK flap (B).
How would you proceed?
—Case prepared by Morgan R. Godin, MD; Nicole Fuerst, MD; and Melissa B. Daluvoy, MD

MARK S. GOROVOY, MD
IFS presents as elevated IOP with fluid accumulation in the LASIK flap interface as well as falsely low IOP readings. Primary endothelial failure typically causes diffuse corneal edema without interface fluid, and IOP readings are accurate. Clearly, this patient is an example of the former scenario, and she was most likely misdiagnosed three times as suffering from primary DSAEK failure. Her history of three glaucoma procedures is highly unusual, especially the exchange of shunts and, finally, the shunt’s explanation for a cyclodestructive procedure.
My recommendation is first to obtain an accurate IOP reading, and to do so I would use the »Model 30 Pneumatonometer (Reichert Technologies). I would expect the IOP to be very high. It would be imperative to address the elevated IOP before pursuing further corneal evaluations or procedures.
Assuming that the IOP is high, my preference would be to place a Baerveldt 350-mm2 glaucoma implant in the inferior quadrant. Once the IOP had been controlled for 6 weeks, allowing the tube ligature time to dissolve, it would then be possible to assess the patient’s DSAEK status accurately. Normalization of the IOP should eliminate the LASIK flap cleft and, I would hope, allow verification that the DSAEK graft was viable after the cornea had cleared. Under no circumstances should further corneal surgery be performed before the IOP normalizes.

RICHARD S. HOFFMAN, MD
Before assessing the next step for this patient, it would be essential to learn how her IOP was calculated. Measuring IOP with either applanation tonometry or a »Tono-Pen (Reichert Technologies) over the central cornea yields an artificially low value in eyes with IFS.1 Patients with IFS can be misdiagnosed as having diffuse lamellar keratitis and treated aggressively with topical steroids, only to experience worsening optic nerve damage because of undiagnosed steroid-induced glaucoma in the presence of interface fluid.4
In this case, if the IOP has been measured centrally, over the area of interface fluid, the actual IOP may be above 40 mm Hg as opposed to 19 mm Hg, and the patient may be losing vision from glaucoma as well as from the decompensated cornea. For that reason, in the presence of interface fluid, all IOP measurements should be taken with a Tono-Pen in the peripheral cornea. IFS can result from corneal endothelial dysfunction (a failed graft) or from elevated IOP; an accurate pressure reading will assist with the differential diagnosis.
Because of this patient’s problematic history with topical glaucoma therapy, if her IOP is dangerously high, I would recommend that she undergo a repeat tube shunt procedure. My hope would be that this intervention would help to resolve the IFS. Unfortunately, if IFS is allowed to persist for months, many patients will develop permanent haze in the flap interface from keratocyte hydropic degeneration.2 Ultimately, these patients will require full-thickness corneal transplantation to resolve both the stromal haze and any endothelial failure.5

ROBERT K. MALONEY, MD
The key features of this case are a compromised endothelium from a DSAEK procedure, fluid collected in the original LASIK flap interface, an IOP apparently in the high normal range, and a hazy cornea. IFS develops when aqueous crosses the endothelium and collects in the potential space that is the LASIK flap interface. Aqueous can cross the endothelium either because elevated IOP drives it across or because endothelial compromise prevents adequate drying of the stroma through the normal endothelial pump function. This case appears to have features of both mechanisms: the endothelial cell density is reduced after DSAEK, and the patient has a history of steroid-induced glaucoma and is presumably still taking steroids because of the DSAEK.
When my colleagues and I originally described IFS,1 we emphasized the difficulty of accurately measuring IOP in patients with this condition. In eyes with a fluid pocket, applanation tonometry measures the pressure in the pocket rather than in the eye itself. Accurately measuring IOP requires the use of a Tono-Pen on the peripheral cornea, outside the area of the fluid pocket. We reported on eyes that had IOPs of less than 10 mm Hg when measured centrally by applanation tonometry but 35 mm Hg or higher when measured on the peripheral cornea. Three of the six eyes in our original report had severe glaucomatous optic atrophy because of this unrecognized elevated IOP. Figure 3 appears to show diffuse microcystic edema, which suggests that the true IOP is significantly higher than indicated.
Draining the fluid in this case did not resolve the fluid pocket because it did not alter the fundamental physiology. This patient needs to have her IOP reduced to the lowest possible level. An IOP measurement on the peripheral cornea will reveal whether the pressure in the eye is elevated. If so, a replacement tube with aggressive lowering of the IOP, even at the expense of a large bleb, is indicated. With a true IOP of 15 mm Hg or less and a somewhat functional DSAEK graft, the interface fluid pocket should resolve on its own.

ALAN N. CARLSON, MD
The frequency with which I am seeing late-onset problems with LASIK flap interfaces is increasing. Several key features of this case are notable. Most noteworthy is that the LASIK flap interface, despite time and healing, apparently remains susceptible to fluid collection if the IOP is elevated, particularly when there is endothelial compromise. Also worth mentioning is that inflammation can occur in the interface with trauma or surgery, in this case causing late-onset diffuse lamellar keratitis.
I see more of these cases today than in the past, I believe, because aging LASIK patients develop problems requiring surgery—cataracts, glaucoma, and an increased risk of developing retinal detachment associated with axial myopia. It thus becomes more relevant for cataract, glaucoma, and vitreoretinal surgeons to ascertain whether or not a patient has ever undergone LASIK.
I would also point out that vitreoretinal surgeons who have a low threshold for debriding the corneal epithelium to improve intraoperative visualization are more likely to encounter healing problems owing to the LASIK flap. Surface healing may be slower due to flap innervation and relative neurotrophism, and fluid may collect in the interface because of only moderately elevated IOP, which, as the panel noted, can then be challenging to measure accurately.
1. Hamilton DR, Manche EE, Rich LF, Maloney RK. Steroid-induced glaucoma after laser in situ keratomileusis associated with interface fluid. Ophthalmology. 2002;109(4):659-665.
2. Dawson DG, Schmack I, Holley GP, Waring GO 3rd, Grossniklaus HE, Edelhauser HF. Interface fluid syndrome in human eye bank corneas after LASIK: causes and pathogenesis. Ophthalmology. 2007;114(10):1848-1859.
3. Randleman JB, Shah RD. LASIK interface complications: etiology, management, and outcomes. J Refract Surg. 2012;28(8):575-586.
4. Galal A, Artola A, Belda J, et al. Interface corneal edema secondary to steroid-induced elevation of intraocular pressure simulating diffuse lamellar keratitis. J Refract Surg. 2006;22(5):441-447.
5. Hoffman RS, Fine IH, Packer M. Persistent interface fluid syndrome. J Cataract Refract Surg. 2008;34(8):1405-1408.




