Marguerite McDonald, MD, FACS, headed the research team that investigated the use of the excimer laser for the correction of optical errors. When an opportunity arose to treat the first sighted human patient, she had the honor of performing that PRK procedure. March 25, 2018, marked the 30th anniversary of this exciting and disruptive procedure, which was born in the laboratory and went on to make a name for itself in popular culture. Here, Dr. McDonald talks to CRST about the past, present, and future of laser vision correction.
CRST: Looking back, were there moments when it seemed that laser vision correction was no more than a dream that was doomed to fizzle? What was the key to keeping the dream alive?
Marguerite McDonald, MD, FACS: There were absolutely some dark days and setbacks. Our team, which initially comprised Charles R. Munnerlyn, PhD; Stephen L. Trokel, MD; and me—and was soon thereafter joined by Stephen D. Klyce, PhD—started by ablating thousands of plastic discs, then cadaver animal eyes, then cadaver human eyes, then living rabbits and monkeys, using an early prototype of the Visx laser (now Johnson & Johnson Vision). We started with a crude, five-step ablation for myopia that was created by a closing diaphragm. I would shoot, close the diaphragm with a hand crank to the next position, then shoot again, and repeat until the fifth and smallest diaphragm position had been utilized. The living rabbits were, at first, positioned several feet away from the industrial excimer laser we were using. They nearly all healed with thick, white, elevated hyperplastic scars. We had no idea why this was happening or if the problem was fixable. About that time, during the same week, my research coordinator and technician quit; they both said the same thing: It was too depressing to work on such a loser of a project. The cornea fellow left the excimer team shortly thereafter for the same reason.
We then made an intuitive leap: Maybe if the ablations were smoother, there would be less scarring. The excimer laser was modified so that there were now 40 positions to the closing diaphragm instead of only five, and the process was automated; in other words, the hand crank disappeared. It seems so obvious now that smoother ablations are better, but we were in completely unknown territory at that time. Smoother was indeed better: The hyperplastic scars disappeared, and the outcomes were good enough for us to move to monkeys. There were, of course, other modifications, such as to the laser, to the algorithms, and to the perioperative regimen, but the move to smoother ablations, with more diaphragm positions, was an important turning point in the project.
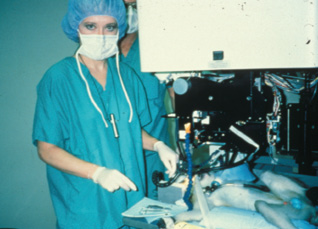
Figure 1. Dr. McDonald preparing to do PRK on a monkey; an early prototype of the Visx excimer laser is shown.
We were still doing monkey trials (Figure 1) when we met Alberta Cassady, a 62-year-old woman with cancer of the orbit requiring an exenteration. Though she was facing a disfiguring operation with a poor prognosis, she asked if anyone wanted to do an experiment on her normal, healthy eye before it was removed. As time was of the essence, we got emergency permission from the FDA to bring Mrs. Cassady to the Delta Primate Center (the Tulane-owned vivarium that LSU Eye Center was then using) in Covington, Louisiana, for PRK. We literally rushed her past the monkey cages to the laser lab, and on March 25, 1988, I had the honor of performing the first laser vision correction procedure on the normal eye of a living human patient. We examined Mrs. Cassady daily and obtained the specimen 11 days later, at the time of her exenteration. The refractive and visual results, along with the beautiful healing pattern seen on pathology, gave the FDA the confidence to let us leapfrog ahead to the blind eye study in humans (Figure 2).1 Mrs. Cassady’s unique case saved us many months, perhaps years, in the development of laser vision correction, and we were excited to bring the excimer laser across Lake Pontchartrian to the LSU Eye Center in New Orleans.
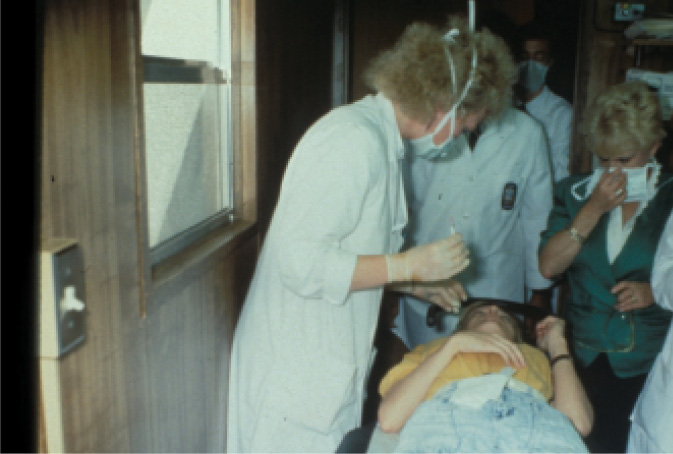
Figure 2. Dr. McDonald in the excimer laser trailer at LSU Eye Center, about to give a retrobulbar injection to a patient in the blind-eye study. Eye trackers did not exist yet; retrobulbar anesthesia was one of the methods used to fixate the eye.
The university was afraid that the laser would leak argon fluoride gas and kill everyone, however, so the excimer was placed in a trailer outside the building. We were disappointed, and also somewhat embarrassed to bring patients to the trailer. It turned out to be a serendipitous occurrence, however, as the trailer was only a few feet away from the LSU trash compactor. Every time the compactor was operating, the trailer shook gently. We started to notice that patients who had been treated while the compactor was in action did better than those who had been treated when the compactor was at rest. The trash compactor was quite critical to our success; we got much smoother ablations than we deserved to get. Much later, of course, the diaphragm delivery system was replaced by a flying spot (which was only possible when eye trackers were also simultaneously introduced), which made the ablations that much smoother.
Not long after her exenteration, and during our blind eye clinical trial, Mrs. Cassady lost her battle with cancer. Though the university’s policy was to name buildings or laboratories only after rich donors, we insisted that the excimer laboratory—the trailer—be named after this extraordinarily brave and selfless woman. Hence, the trailer was named the Alberta H. Cassady LSU/Lions Excimer Laser Laboratory.
As we spoke about and published our early research on animals and humans, there was a good deal of anti–refractive surgery sentiment in the ophthalmic community. RK had always been controversial, and there were early reports emerging of the slow hyperopic shift that was occurring in 50% of the post-RK patients. This was later confirmed by the PERK study’s 10-year report.2 Many of the top names in ophthalmology felt that refractive surgery was frivolous at best, and unethical at worst. There was even a vitriolic diatribe against me and laser refractive surgery that was written by a respected and internationally renowned ophthalmologist and published in a leading ophthalmic journal. It was a difficult time, as I was a 30-something female at a southern state university, LSU, and the author was one of the most famous and respected ophthalmologists in the world.
CRST: Laser vision correction is now ubiquitous. Your contribution to science has essentially altered society, in that people who were once hampered by a dependence on eyeglasses now have expanded options. How do feel about playing a pivotal role in bringing to fruition this procedure that improves peoples’ lives?
McDonald: It was and is a great honor to have played a role in developing an option for those who want to safely eliminate their refractive errors (Figure 3). We felt at the time that we were doing important work, but we couldn’t possibly foresee that people all over the world would embrace it as they have. It is thrilling to meet people every day—in the office, at airports, in restaurants, at the post office, wherever—who mention that they have had laser vision correction and how happy they are. When they find out that I am an eye surgeon, they ask, “Hey, do you do that surgery to get rid of glasses?” It always makes me smile.

Figure 3. Dr. McDonald (seated) and Dr. Klyce (standing above Dr. McDonald) during a PRK in the partially sighted and sighted eye trials, which were the first to be done in the LSU Eye Center building. By now, the Visx laser was starting to look like a commercial model.
CRST: Since performing the inaugural excimer laser vision correction procedure, you have remained at the forefront of the increasingly sophisticated spinoff procedures, such as wavefront-based excimer surgery, epi-LASK, and epi-Bowman keratectomy. What do you think the future holds for laser vision correction, from a clinical and cultural perspective?
McDonald: We will, as a specialty, continue to improve refractive surgery; that is the nature of surgeons, and ophthalmologists in particular. We never say, “These results are good enough,” and rest on our laurels, and we are fortunate to have corporate partners that feel the same. I love that about all of us.
I do think that the time will shortly come when refractive surgery is a rite of passage for the middle class: braces at age 12; contact lenses at age 13; refractive surgery after the last year of school, somewhere between ages 21 and 26; premium cataract surgery at age 60 to 65.
Hopefully, refractive surgery will become commonplace in the third world as well. According to the World Health Organization, the major global cause of moderate to severe vision impairment is uncorrected refractive error (53%).3 So our goals are to continue to improve refractive surgery and to assist in its dissemination.
1. McDonald MB, Frantz JM, Klyce SD, et al. Central photorefractive keratectomy for myopia. The blind eye study. Arch Ophthalmol. 1990;108(6):799-808.
2. Waring GO 3rd, Lynn MJ, McDonnell PJ. Results of the prospective evaluation of radial keratotomy (PERK) study 10 years after surgery. Arch Ophthalmol. 1994;112(10):1298-1308.
3. Vision impairment and blindness. World Health Organization. October 2017. www.who.int/mediacentre/factsheets/fs282/en. Accessed March 19, 2018.




