
The emergence of microinvasive glaucoma surgery (MIGS) has allowed cataract and refractive surgeons to treat glaucoma earlier and more safely compared with filtration surgery. Because MIGS procedures are mostly performed ab interno, they will not interfere with cataract surgery planning or induce astigmatism at a later date. MIGS also gives patients an opportunity to reduce their dependence on topical medication, agents that negatively affect the ocular surface, so quality of vision can improve.
This article provides a brief update on current and future MIGS options that fit into cataract and refractive surgeons’ toolboxes.
CURRENT OPTIONS
Trabecular Meshwork
iStent Trabecular Micro-Bypass Stent
The iStent Trabecular Micro-Bypass Stent (Glaukos) drains aqueous flow into Schlemm canal from the anterior chamber, effectively bypassing the damaged or blocked trabecular meshwork (see video). The device has proven to be effective. Ferguson and colleagues recently analyzed iStent placement with cataract surgery in 350 eyes with open-angle glaucoma. In the study, mean IOP decreased from 19.13 ±6.34 mm Hg preoperatively to 15.17 ±3.53 mm Hg (P < .0001) 2 years postoperatively, and the mean number of medications decreased from 1.19 ±1.00 preoperatively to 0.61 ±0.96 (P < .0001).1
Michael Greenwood, MD, implants the iStent Trabecular Micro-Bypass Stent.
These researchers reported similar findings in a study population with pseudoexfoliative glaucoma.2 The investigators also found the iStent to be effective in pseudophakic patients, with the IOP decreasing from 20.26 ±6.00 mm Hg preoperatively to 13.62 ±4.55 mm Hg postoperatively (P < .01) and the number of medications reduced from 1.95 ±1.01 preoperatively to 1.69 ±1.28 (P >0.05) 1 year postoperatively.3
Although the iStent is currently only FDA approved to be used in combination with cataract surgery, the implant can be used in a variety of situations.
Ab Interno Canaloplasty
Ab interno canaloplasty (ABiC) involves placing a microcatheter (iTrack 250A; Ellex) into Schlemm canal and threading it for 360º, which permits complete access to the collector channels without leaving behind any devices. ABiC can be performed as a standalone procedure or in combination with cataract surgery (see video).
Mahmoud A. Khaimi, MD, demonstrates ab interno canaloplasty to restore the aqueous outflow pathway.
In a recent study of 130 patients who underwent ABiC in combination with cataract surgery, mean IOP decreased from 17.1 ±5.0 to 13.1 ±2.1 mm Hg at 12 months (n = 34).4 The mean number of medications was 2.0 ±1.0 at baseline and decreased to 1.0 ±1.0 at 12 months. Ninety-eight patients who underwent ABiC as a standalone procedure had a preoperative IOP of 21.5 ±7.4 mm Hg at baseline. Twelve months after surgery (n = 14), mean IOP was reduced by 36.74% to 13.6 ±1.20 mm Hg. The mean number of medications dropped from 3.0 ±1.0 at baseline to 1.0 ±1.0 at 12 months.
Trabectome
Approved by the FDA in 2006, the Trabectome (NeoMedix) was the first MIGS procedure. The surgeon uses microelectrocautery to ablate the trabecular meshwork and inner wall of Schlemm canal. Trabectome surgery may be performed independently or in conjunction with other intraocular procedures such as cataract extraction and IOL placement (see video). Researchers recently reported the 10-year outcomes of 5,435 cases (2,250 eyes underwent ab interno trabecular ablation plus phacoemulsification).5 Ninety months postoperatively, IOP had decreased from an average preoperative level of 23.0 ±7.9 to 16.5 ±3.8 mm Hg (P < .01), and the number of glaucoma medications had dropped from 2.6 ±1.3 to 1.6 ±1.3 (P = 1.00). These results represent an approximately 30% reduction in IOP from baseline, with a significant decrease in medication reliance.
Sameh Mosaed, MD, combines Trabectome surgery with cataract extraction.
Kahook Dual Blade
The Kahook Dual Blade (New World Medical) removes the trabecular meshwork tissue more completely than a simple incision; it surgically creates a cleft more resistant to closure and produces more sustained IOP control.6 Major advantages of the procedure are that it can be performed alone or simultaneously with cataract surgery, it requires no additional materials or equipment, and it can be used in cases of mild to severe glaucoma (see video).
John Berdahl, MD, presents his goniotomy technique using the Kahook Dual Blade.
In a recent study, 71 eyes underwent cataract surgery combined with the Kahook Dual Blade procedure. The baseline IOP in this group was 17.4 ±5.2 mm Hg, and the mean number of hypotensive medications was 1.6 ±1.3 preoperatively. Nine months postoperatively, the mean IOP was 12.4 ±3.4 mm Hg, a decrease of 5 mm Hg or 28.7% (P < .001), and the number of medications decreased to 0.6 ±0.8 (P = .005).7
Trab360
The Trab360 (Sight Sciences) allows surgeons to unroof 360º of the trabecular meshwork. The ophthalmologist makes a small incision in the trabecular meshwork, advances the probe of the device into Schlemm canal for 180º, and then performs a push-pull motion to unroof the canal. The maneuver is then repeated in the other direction (see video).
In this episode of Glaucoma Today Journal Club, Steven Sarkisian Jr, MD, explains how to perform ab interno trabeculotomy with the Trab360.
In a small study of 26 patients with a mean follow-up period of 131.5 ±101.6 days, the IOP measured 19.8 ±6.4 mm Hg preoperatively, and the mean number of medications was 1.1 ±1.2.8 At final follow-up, the mean IOP was 13.5 ±4.6 mm Hg, and the mean number of medications was 0.2 ±0.5, with 19 patients (73%) requiring no medication.
Supraciliary Space
CyPass Micro-Stent
Instead of the traditional pathway of aqueous flow, the CyPass Micro-Stent (Alcon) targets the alternative uveoscleral pathway and allows aqueous to flow from the anterior chamber to the supraciliary space. The device is approved for use in conjunction with cataract surgery (see video).
Russell Swan, MD, implants the CyPass Micro-Stent.
In a multicenter, interventional, randomized clinical trial of patients who underwent cataract surgery plus placement of the CyPass versus cataract surgery alone, mean IOP decreased by 7.4 mm Hg in the microstent group compared to 5.4 mm Hg in the control group (P < .001).9 Sixty-one percent of the microstent group and 44% of the control group had an unmedicated IOP between 6 and 18 mm Hg. In addition, 85% of microstent subjects versus 59% of the control subjects were free of medication postoperatively.
Subconjunctival Space
Xen Glaucoma Treatment System
The Xen Glaucoma Treatment System (Allergan) aims to lower IOP by creating a subconjunctival drainage pathway. Early this year, the FDA approved the device as a standalone procedure for refractory glaucoma. Prior to implanting the stent, the surgeon places an antimetabolite medication such as mitomycin C in the subconjunctival space. The device is inserted via a needle injector system that passes from the anterior chamber through the trabecular meshwork and sclera, after which it exits under the conjunctiva. The stent is then deployed. It allows aqueous to flow from the anterior chamber to the subconjunctival space (see video).
Dr. Greenwood shares his first Xen case.
Researchers recently reported the results of a study of 41 eyes of 33 patients with open-angle glaucoma who underwent implantation of the Xen in combination with cataract surgery.10 The mean preoperative IOP was 22.5 ±3.7 mm Hg on 2.5 ± 0.9 medications. After 12 months, the mean postoperative IOP measured 13.1 ±2.4 mm Hg (mean IOP reduction of 41.82%) with a mean of 0.4 ±0.8 medications.
FUTURE OPTIONS
Trabecular Meshwork
iStent Inject
The iStent Inject is the second-generation trabecular meshwork bypass stent from Glaukos. The device is currently in phase 3 FDA trials. During this MIGS procedure, two devices are placed in the trabecular meshwork.
In a small study, 20 patients underwent cataract surgery plus implantation of two iStent Inject devices and were observed for 47.4 ±18.46 months.11 Mean baseline IOP measured 19.95 ±3.71 mm Hg with medication and 26 ±3.11 mm Hg after washout. Mean end follow-up IOP was 16.25 ±1.99 mm Hg, representing a reduction of 36.92% or 9.74 ±3.14 mm Hg (P < .001) from baseline washout IOP. The mean number of medications decreased significantly from 1.3 ±0.66 to 0.75 ±0.79 (P = .017). Forty-five percent of patients were free of medication by the end of follow-up.
Hydrus Microstent
Currently in phase 3 FDA trials, the Hydrus Microstent (Ivantis) is a crescent-shaped trabecular bypass device that is 8 mm long and straddles 3 clock hours of Schlemm canal (see video). The aim is to access a greater number of collector channels than could be accessed via a smaller device and to dilate the canal. The device acts as a scaffold and does not block the collector channel ostia. Researchers studied 100 patients who underwent cataract surgery alone or in combination with the Hydrus procedure and were observed for 24 months.12 A significantly greater proportion of the combined patients reached the endpoint of a 20% reduction in diurnal IOP (80% vs 46%, P = .0008). The IOP was also significantly lower in the combined group (16.9 ±3.3 vs 19.2 ±4.7 mm Hg, P = .0093). A much higher number of patients in the combined group achieved a decrease in ocular hypotensive medication (73% vs 38%, P = .0008).
In this episode of CRST Journal Club, Cathleen McCabe, MD, joins James Katz, MD, to share data on the Hydrus Microstent and to describe the device and its implantation.
Supraciliary Space
iStent Supra
Limited data are available on the iStent Supra (Glaukos), which is in phase 3 FDA trials. Like the CyPass, the iStent Supra targets the supraciliary space (see video). Forty-two patients underwent implantation of the device as a standalone procedure.13 The mean preoperative IOP was 20.4 ±2.4 mm Hg, with all patients on two medications, and 24.8 ±3.4 mm Hg after washout. Postoperatively, all patients were placed on travoprost, and their mean IOP measured 13.2 mm Hg or lower at 1 year. Ninety-eight percent of the eyes were considered as success (decrease in IOP of ≥20% with a reduction of one medication). In the remaining eye, the IOP measured 18 mm Hg, with a reduction of one medication. Furthermore, 90% of patients achieved a postoperative IOP below 15 mm Hg with a reduction of one medication.
John Berdahl, MD, implants the iStent Supra in a patient with a history of Trabectome surgery.
Subconjunctival Space
InnFocus MicroShunt Drainage Device
The InnFocus MicroShunt Drainage Device (Santen; Figure) is an ab externo subconjunctival glaucoma device. Compared with other MIGS procedures, implantation of the MicroShunt involves more steps akin to trabeculectomy. Surgery requires a peritomy and dissection of a scleral pocket to allow insertion of an 8.5-mm-long tube with a 70-µm inner lumen under the limbus into the anterior chamber. Intraoperative antifibrotics are used, as during trabeculectomy.
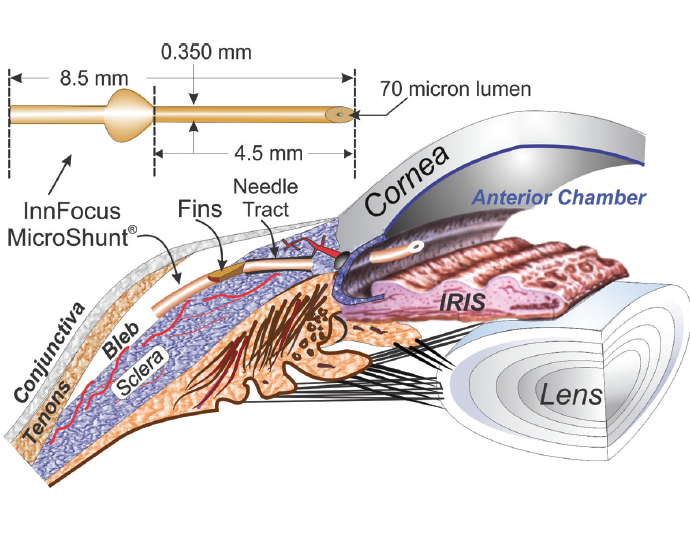
(Courtesy of Santen.)
Figure. The InnFocus MicroShunt is an ab externo drainage device.In a nonrandomized study of 23 patients who underwent placement of the MicroShunt with or without cataract surgery, overall qualified success (IOP ≤14 mm Hg and IOP reduction ≥ 20%) was 95% at 3 years.14 Mean IOP decreased from 23.8 ±5.3 mm Hg at baseline to 10.7 ±3.5 mm Hg at 3 years, and the mean number of medications per patient was reduced from 2.4 ±0.9 to 0.7 ±1.1.
CONCLUSION
MIGS procedures continue to be developed to permit the simultaneous treatment of cataracts and glaucoma. More data become available each month. The future is bright for both patients and surgeons.
1. Ferguson TJ, Berdahl JP, Schweitzer JA, Sudhagoni RG. Clinical evaluation of a trabecular microbypass stent with phacoemulsification in patients with open-angle glaucoma and cataract. Clin Ophthalmol. 2016;10:1767-1773.
2. Ferguson TJ, Swan R, Ibach M, et al. Trabecular microbypass stent implantation with cataract extraction in pseudoexfoliation glaucoma. J Cataract Refract Surg. 2017;43(5):622-626.
3. Ferguson TJ, Berdahl JP, Schweitzer JA, Sudhagoni RG. Evaluation of a trabecular micro-bypass stent in pseudophakic patients with open-angle glaucoma. J Glaucoma. 2016;25(11):896-900.
4. Khaimi M. Twelve-month follow-up of ab interno canaloplasty as a standalone treatment and in adjunct to cataract surgery for the treatment of primary open-angle glaucoma. Poster presented at: The 27th Annual AGS Meeting; March 9, 2017; Coronado, CA.
5. Mosaed S. The first decade of global Trabectome outcomes. Eur Ophthalmic Rev. 2014;8(2):113-119.
6. Seibold LK, SooHoo JR, Ammar DA, Kahook MY. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am J Ophthalmol. 2013;155(3):524-529.e2.
7. Greenwood MD, Abdullah S, Radcliffe NM, et al. A novel dual blade device for goniotomy: 9 month follow up. Poster presented at: ASCRS/ASOA Congress & Symposium; May 5-9, 2017; Los Angeles, CA.
8. Sarkisian SR, Allen E, Ding K, et al. New way for ab interno trabeculotomy: initial results. Poster presented at: ASCRS/ASOA Symposium & Congress; April 17, 2015; San Diego, CA.
9. Vold S, Ahmed, II, Craven ER, et al. Two-year COMPASS Trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016;123(10):2103-2112.
10. De Gregorio A, Pedrotti E, Russo L, Morselli S. Minimally invasive combined glaucoma and cataract surgery: clinical results of the smallest ab interno gel stent (published online ahead of print May 29, 2017). Int Ophthalmol. doi:10.1007/s10792-017-0571-x.
11. Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, et al. Glaukos iStent inject trabecular micro-bypass implantation associated with cataract surgery in patients with coexisting cataract and open-angle glaucoma or ocular hypertension: a long-term study. J Ophthalmol. 2016;2016:1056573.
12. Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. A randomized trial of a Schlemm’s canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology. 2015;122(7):1283-1293.
13. Jünemann A. Twelve-month outcomes following ab interno implantation of suprachoroidal stent and postoperative administration of travoprost to treat open angle glaucoma. Poster presented at: XXXI Congress of the ESCRS; 2013; Amsterdam, The Netherlands. Available at: http://escrs.org/amsterdam2013/programme/posters-details.asp?id=19512. Accessed August 16, 2017.
14. Batlle JF, Fantes F, Riss I, et al. Three-year follow-up of a novel aqueous humor microshunt. J Glaucoma. 2016;25(2):e58-e65.




