
The skill of intraoperative gonioscopy is not a simple task of docking a surgical goniolens onto the corneal surface. Here is my advice on how to optimize outcomes while minimizing complications during microinvasive glaucoma surgery (MIGS).
OR SETUP
Prior to starting angle surgery, raise both the stretcher and the surgical stool while maintaining an upright posture (Figure 1A). With head and microscope tilt, an increased working distance between the oculars and the nasally displaced surgical field will result. To maintain focus, lower both the chair and microscope while keeping a straight back, and raise the eyepiece to maintain primary gaze (Figure 1B). Initially setting the stool in its lowest position will cause unnecessary strain from both neck and back flexion.
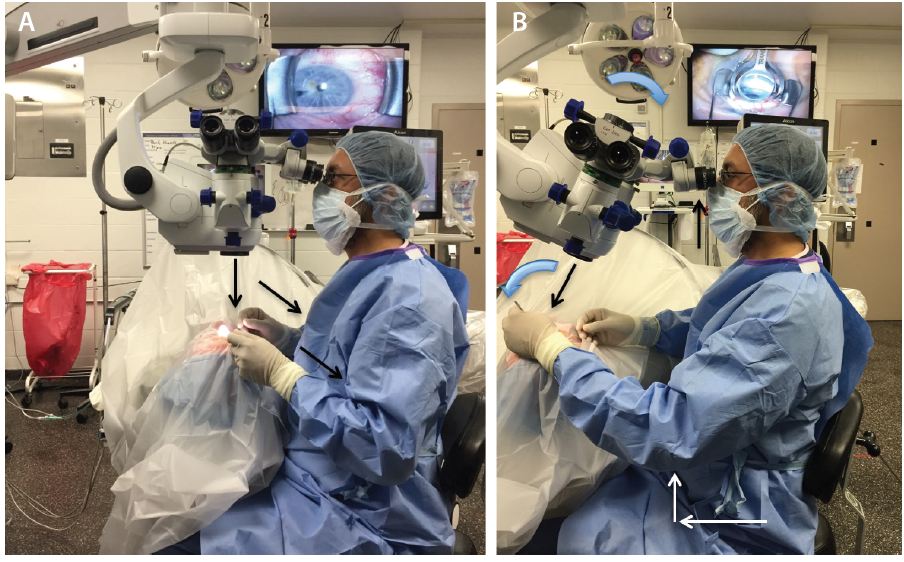
Figure 1. In primary phaco position, the elbows straddle the flanks (oblique arrows), the back is erect, and the coaxial light is perpendicular to the ocular surface (vertical arrow; A). With head and microscope tilt (curved arrows), working distance between the oculars and the surgical field increases (black arrows), and upright posture is maintained. Note that the elbow of the nondominant hand (white arrows) extends anteriorly from the flank during goniolens docking (visible on the TV screen; B).
Avoid using the foot pedal to refocus on the ocular surface and subsequently in the angle under gonioscopic view. Instead, manually lower the microscope each time to bring the target tissue plane into view, thereby preserving the range of focus during angle surgery. It is essential to find the right balance during head and microscope rotations to ensure that surgical instruments approach the target tissue en face parallel to the iris plane. With the patient in a supine position (Figure 1A), the optical axis of the surgical microscope is perpendicular to the eye, denoted by a vertical bar aligned with a horizontal line on the carrier arm in the Zeiss Opmi Lumera 700 system (Carl Zeiss Meditec; Figure 2A). To facilitate surgical flow and minimize awkward body positioning that may cause stress or injury, place a mark or adhesive tape on the carrier arm (Figure 2A and B) to serve as a reference point for your preferred angle tilt. You can quantify the exact rotation of the microscope head in degrees from the vertical using the iOS Compass on the iPhone (Apple; Figure 2C). This will enable OR personnel to rotate the knob on the carrier arm clockwise to bring the microscope into position for optimal viewing of angle structures when coupled with nasal head rotation of approximately 30º to 35º until the coaxial light is aligned along the iris plane.
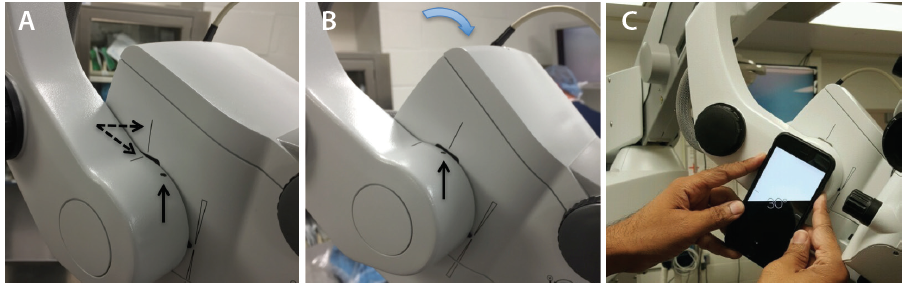
Figure 2. Alignment of the vertical bar on the microscope head with the horizontal line on the carrier arm (dashed arrows; A). The reference point (vertical arrow) denoting the surgeon’s preferred angle tilt is aligned with the vertical bar after microscope rotation (B). Quantification of angle tilt with iOS Compass on an iPhone 6; 30º is displayed (C).
PHACOEMULSIFICATION VERSUS MIGS
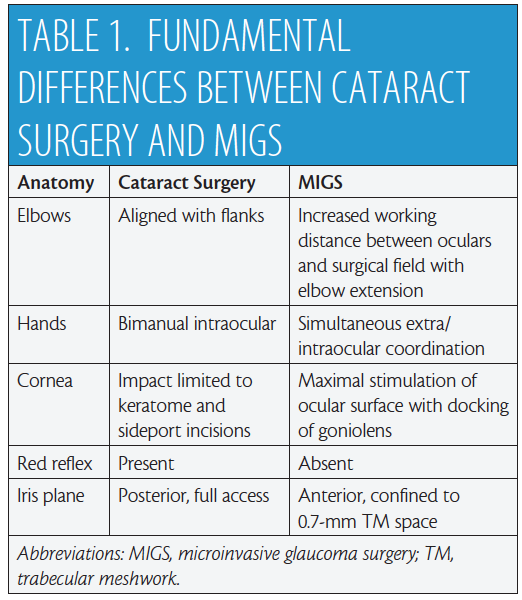
There are fundamental differences between MIGS and cataract surgery (Table 1). In the primary phaco position (Figure 1A), the elbows are straddling the flanks. After head and microscope tilt, the increased working distance anteriorly displaces the elbow of the nondominant hand when the goniolens is docked onto the corneal surface (Figure 1B and video). MIGS requires simultaneous extra- and intraocular coordination of the goniolens and instruments, respectively, making it essentially a one-handed surgery. Angle surgery is confined anterior to the iris plane with a trabecular space approximately one-third (0.7-mm width)1 of a standard keratome incision (2.2-2.4 mm).
The cornea is a highly innervated structure with multiple subepithelial nerve endings.2 During cataract surgery, keratome and sideport incisions induce minimal corneal stimulation. In contrast, there is maximal stimulation of the ocular surface and perilimbal area after goniolens docking (Figure 1B), with potential risk for intraocular complications (iris trauma, bleeding) from involuntary saccades under topical anesthesia.3 Additionally, the absence of a red reflex requires maximizing illumination with appropriate magnification to identify the target tissue.
KERATOME INCISION
Keep in mind two important factors when creating a temporal corneal incision: (1) eccentricity and (2) location (see Keratome Wound Construction and video). I highly recommend using a fixation ring in all cataract surgery cases, not only to gain familiarity with holding the handle of a surgical goniolens but also to access the peripheral cornea by rotating the globe nasally. A leading eye surgeon has recommended initiating the corneal incision at the limbal arcade.4,5 With MIGS, however, there is a potential risk of microbleeds as the keratome advances into the corneal stroma. In such cases, blood subsequently occupies the interface between the goniolens and cornea, obscuring angle viewing. On the other hand, inward displacement of femtosecond laser arcuate incisions from the limbus may cause surgical instruments to interfere with the overlying goniolens at the wound entry site during angle surgery.
KERATOME WOUND CONSTRUCTION
When preparing for surgical gonioscopy, two factors in the creation of a temporal corneal incision are important.
No. 1. ECCENTRICITY
Avoid limbal vasculature, because blood will obscure angle viewing.
- Femtosecond laser arcuate incisions displaced from the limbus can cause friction between instruments and the goniolens.
- In standalone microinvasive glaucoma surgery or precataract combined cases, keratome incisions that are 2 mm or smaller can be made.
No. 2. LOCATION
An incision along the 3- to 9-o’clock plane provides equidistant access to the superior/inferior nasal angle and serves as a pivot/anchor point during surgical manipulation. The surgeon can make additional incisions supero- or inferotemporally to directly access the infero- or superonasal angles.
Manual entry just inside the limbus can avoid these two extremes. As a standalone procedure or when MIGS precedes cataract surgery in combined cases, you can create small keratome incisions (≤ 2 mm) with minimal alteration of ocular integrity while maintaining a pristine, uninterrupted view of the angle structures. After angle surgery, reset the surgical microscope to its initial settings prior to proceeding with phacoemulsification. In contrast, if lens extraction precedes angle surgery, certain conditions such as complex or prolonged cataract surgery and vitrectomy can make performing MIGS challenging owing to poor angle visualization.3,6
Creating the temporal incision along the 3- to 9-o’clock axis offers several advantages, including equidistant access of the nasal angle and a pivot point facilitating angle surgery (see Keratome Wound Construction). Do not hesitate to create additional keratome incisions supero- or inferotemporally if the temporal view is obscured in order to directly access the inferior and superior nasal angle structures, respectively, or if additional clock hours of the angle require treatment based upon the intended MIGS procedure.
GONIOSCOPY
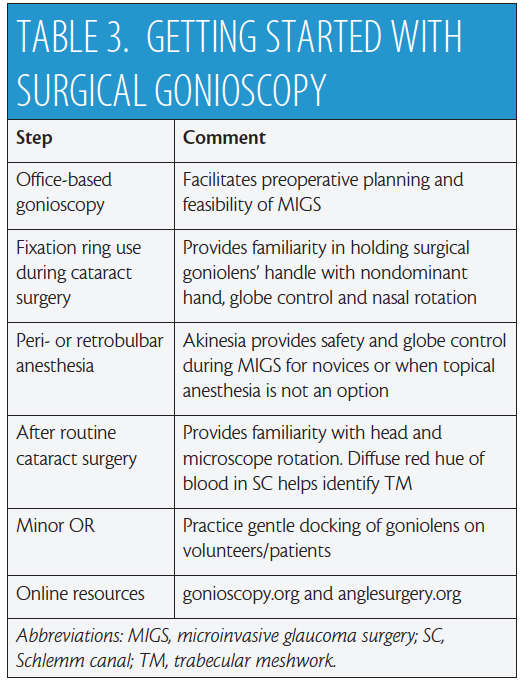
Several surgical goniolenses for MIGS are commercially available. They represent a modification of the Swan Jacob lens7 whereby the grasping handle is contiguous with the viewing lens. These instruments vary by static field of view, extent of corneal contact, image magnification, and handle length—each with its pros and cons.8 A number of these goniolenses have been specifically designed to enhance globe control,8 including the disposable iPrism Clip view stabilizer (Glaukos; Figure 3). Recently, a two-mirror Ocular Upright 1.3× Surgical Gonioprism (Ocular Instruments) with a static 45º field of view was introduced that redirects the oblique gonio image to the coaxial “primary” phaco position (Figure 1A), eliminating the need for any head or microscope tilt.
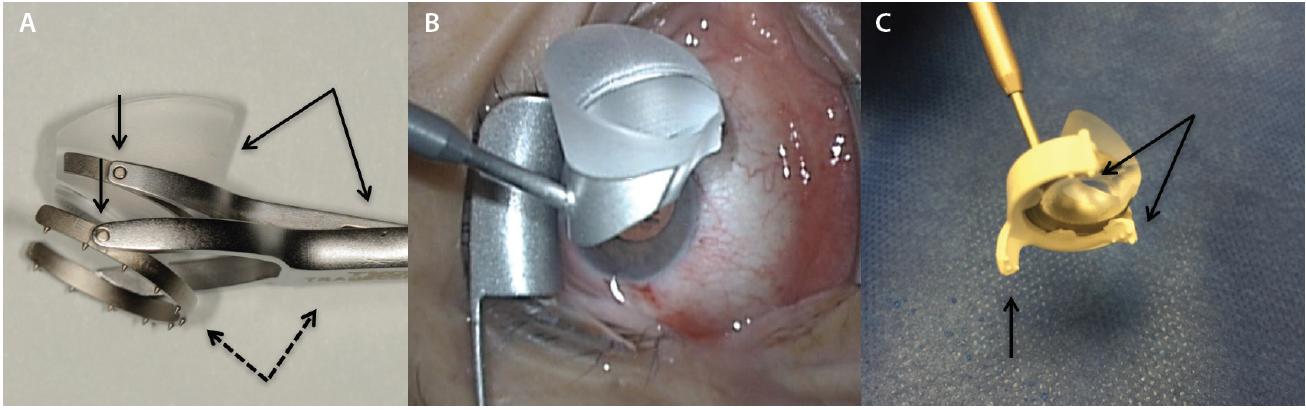
Figure 3. Volk Transcend Vold Gonio (TVG) Surgical Lens (Accutome). The main handle is contiguous with the fixation ring (dashed arrows), while a floating lens is suspended by a separate pendular handle (solid arrows). Multiple pivot points permit surgical adaptability (vertical arrows; A). Ocular Hill Surgical Gonioprism (Ocular Instruments). Note the peripheral flange at the base of the lens to fixate the globe (B). The disposable iPrism Clip view stabilizer is made specifically for the lens base seen in Figure 3B to snap into. Atraumatic, stabilizing surface protrusions (arrows) and extender (vertical arrow) from the clip’s base are designed to stabilize the globe effectively (C).
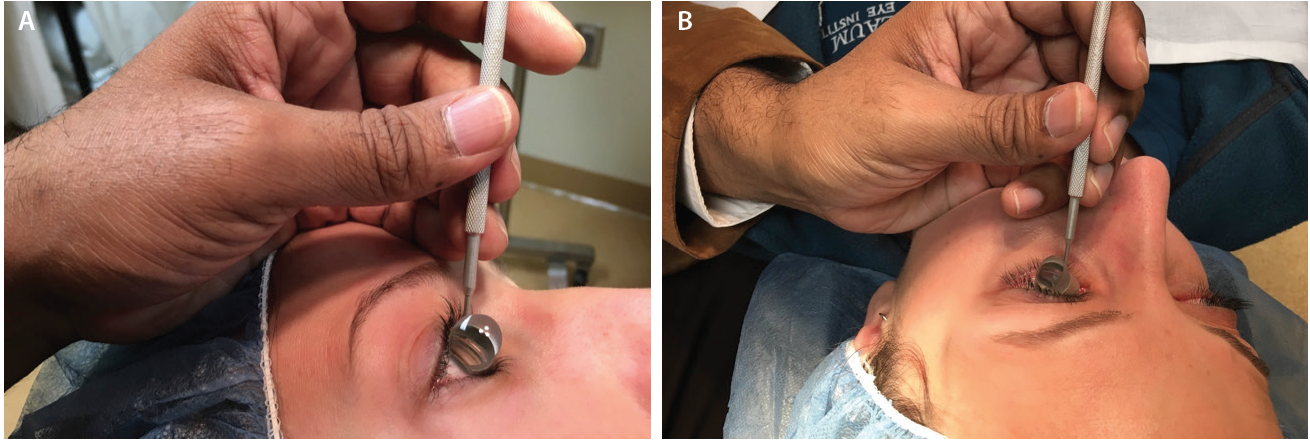
Figure 4. The side of the surgeon’s palm serves as both a weight-bearing anchor and as a pivot point resting on the patient’s forehead (A) or zygoma (B) to help the surgeon dock the goniolens onto the corneal surface with minimal force. Note that the surgeon’s fingers are parallel to the nasal bridge.
As with a writing implement, when the surgical goniolens is held in the nondominant hand, the side of the palm plays two essential roles (Figure 4). First, it functions as a weight-bearing anchor point resting on the patient’s forehead or zygoma based upon laterality. Second, the side of the palm allows for pivoting to gently dock the lens onto the corneal surface with minimal force in order to avoid creating Descemet folds that will obscure angle viewing.
Although an ophthalmic viscosurgical device (OVD) may be used as a coupling medium between the cornea and lens, lidocaine gel not only serves as a topical analgesic but also decreases the sensation of tissue manipulation during surgery.9,10 Additionally, when I use only a cohesive OVD to deepen and stabilize the anterior chamber (AC), particularly in cases that take longer or require multiple manipulations during MIGS, I have found that the OVD tends to egress quickly, resulting in a shallow AC that requires reformation. In my hands, Arshinoff’s soft-shell technique11 provides greater chamber stability with less need to deepen the AC.
If you cannot achieve adequate visualization of the angle, ask the patient to look nasally to bring the target tissue into view. Alternatively, use the surgical goniolens to rotate the globe farther nasally in a controlled manner before embarking on the MIGS procedure.
IDENTIFICATION OF TRABECULAR MESHWORK FOR SCHLEMM CANAL-BASED SURGERY
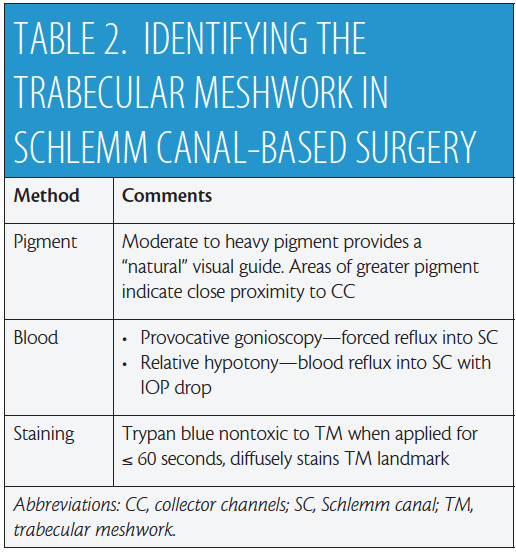
The scleral spur serves as the surgical landmark separating canal-based surgery via the trabecular meshwork (TM) anteriorly from suprachoroid-based surgery via the ciliary body band posteriorly. For surgery based in Schlemm canal (SC), it is critical to assess patients preoperatively with office-based gonioscopy in order to determine if the angle is open or closed (see video).12 Moderate to heavy pigment within the TM can serve as a visual guide during MIGS. Areas of greater pigmentation have been reported to be in close proximity to collector channels, potentially enhancing aqueous outflow,13 but in patients with minimal to no pigment, other methods may be used intraoperatively (Table 2). In standalone MIGS or when the procedure precedes cataract surgery, gentle pressure with the edge of the surgical goniolens on the perilimbal episcleral vasculature nasally will induce regurgitation of blood into SC. Alternatively, gently depressing the posterior lip of the keratome incision to cause the egress of small amounts of viscoelastic will induce a state of relative hypotony. Blood reflux into SC will result when viewed with gonioscopy. At this juncture, pressurizing the AC without overfilling will “freeze” the blood in SC and visually assist you in proceeding with targeted SC-based surgery.
After phacoemulsification, a diffuse red hue usually outlines SC and facilitates MIGS. Trypan blue has been used in ophthalmic surgery for a variety of indications, including cataract surgery, trabeculectomy, and corneal transplantation.14 Cultured human TM cells exposed to commercially available 0.06% trypan blue showed no evidence of toxicity with respect to cell viability after an exposure time of 60 seconds or less.15 At a Glaukos user meeting held on March 3, 2016, Reay Brown, MD, proposed the dye’s application to diffusely stain the TM landmark as a visual guide.
CONCLUSION
Safe, successful MIGS demands intraoperative gonioscopy, which itself requires a number of perioperative steps. Table 3 provides my recommendations for mastering this skill set. Try different goniolenses to identify which best meets your surgical needs, and remember that, on occasion, it may be necessary to use more than one type of surgical lens during MIGS. To learn intraoperative gonioscopy and build your surgical confidence, I recommend making use of educational resources available online.12,16
1. Kasuga T, Chen YC, Bloomer MM, et al. Trabecular meshwork length in men and women by histological assessment. Curr Eye Res. 2013;38:75-79.
2. Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59:263-285.
3. Shareef S. Get in on the ACT—optimization of angle surgery . Eyetube.net. http://bit.ly/2cfezN3. Accessed August 19, 2016.
4. Colvard M. Incisions. In: Colvard M, ed. Achieving Excellence in Cataract Surgery. A Step-by-Step Approach. Encino, CA: self-published; 2009:11-17.
5. Colvard DM. Corneal incision . Eyetube.net. http://bit.ly/2cwmJ3e. Accessed August 18, 2016.
6. Shareef S. Optimizing angle surgery with ACT. Glaucoma Today. May/June 2015;13(3):48-50. http://bit.ly/2cwk0XN. Accessed September 10, 2016.
7. Vold SD, Ahmed IIK. Intraoperative gonioscopy: past, present, and future. Glaucoma Today. Summer 2010;8(3):31-34. http://bit.ly/2ccL38L. Accessed September 10, 2016.
8. Shareef S, Alward W, Crandall A, et al. Intra-operative gonioscopy: a key to successful angle surgery. Expert Rev Ophthalmol. 2014;9:515-527.
9. Page M, Fraunfelder F. Safety, efficacy, and patient acceptability of lidocaine hydrochloride ophthalmic gel as a topical ocular anesthetic for use in ophthalmic procedures. Clin Ophthalmol. 2009;3:601-609.
10. Bardocci A, Lofoco G, Perdicaro S, et al. Lidocaine 2% gel versus lidocaine 4% unpreserved drops for topical anesthesia in cataract surgery. Ophthalmology. 2003;110:144-149.
11. Arshinoff SA. Dispersive-cohesive viscoelastic soft shell technique. J Cataract Refract Surg. 1999;25:167-173.
12. University of Iowa Health Care Ophthalmology and Visual Sciences. www.gonioscopy.org. Accessed August 21, 2016.
13. Hann CR. Fautsch MP. Preferential fluid flow in the human trabecular meshwork near collector channels. Invest Ophthalmol Vis Sci. 2009;50(4):1692-1697.
14. Jhanji V, Chan E, Das S, et al. Trypan blue dye for anterior segment surgeries. Eye (Lond). 2011;25(9):1113-1120.
15. Tsaousis KT, Kopsachilis N, Tsinopoulos IT, et al. Time-dependent morphological alterations and viability of cultured human trabecular cells after exposure to trypan blue. Clin Experiment Ophthalmol. 2013;41(5):484-490.
16. Angle surgery. UR Medicine Flaum Eye Institute. www.anglesurgery.org. Accessed August 21, 2016.




