CASE PRESENTATION
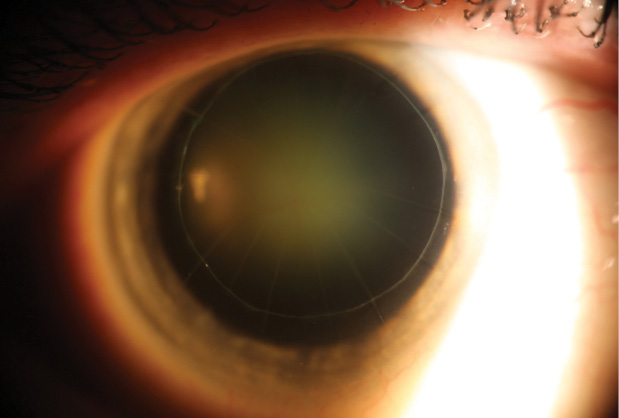
Figure. Nuclear sclerotic cataract in a post-RK eye. The lasso suture is more than 11 years old.
- Corneal considerations taking into account the lasso suture I placed more than 11 years ago to both steepen and stabilize the progressive, ongoing flattening from aggressive RK surgery
- IOL calculations and selection in a postrefractive surgery patient who continues to have high expectations
UDAY DEVGAN, MD
This is certainly a challenging case, and it would be important to set the patient's expectations appropriately. The primary goal in estimating the IOL's power is to avoid a hyperopic postoperative result by choosing the highest-powered lens determined by the methods on the website of the American Society of Cataract and Refractive Surgery (ASCRS; iolcalc.org). If there is any doubt with the calculations, a few diopters can be added to the IOL's power, because it would be best to leave the patient myopic. I would question any calculations that called for an IOL with a power of less than +20.00 D, because the optimal power is likely to be significantly higher. With so many RK cuts, intraoperative aberrometry may not be able to read the eye or produce reasonable calculations.
The phaco incision should be a scleral tunnel to avoid intersecting with the RK cuts. The lasso suture is beautifully placed and has served the patient well, but although it has held up well for more than a decade, it will not last forever. I would avoid touching the lasso suture during the cataract surgery and instead closely monitor the eye in the postoperative period. In the unlikely event that the patient ended up with too much postoperative myopia, the lasso suture could be cut to flatten the cornea, or it could be replaced by a suture with less tension.
When observing the patient after cataract surgery, it will be important to remember that many months may elapse before the cornea returns to its preoperative state and the refraction stabilizes. Ultimately, the patient will do well and be happy with the increase in vision achieved through removing the cataract and treating a large part of her refractive error.
KEVIN M. MILLER, MD
Cataract development in a post-RK eye is something all of us see on a regular basis. What makes this patient unique is the lasso suture.
Because the cornea is presumably stable and the suture has been quiet for more than 11 years, I would leave it alone. I would not want to risk a hyperopic corneal shift or the corneal instability that might result from the suture's removal.
I would obtain corneal topography or a Scheimpflug map and place my phaco incision on the steep axis of corneal astigmatism. Because there are more than eight keratotomy incisions, I would not attempt operating in the clear cornea. Instead, I would move the cataract incision back to the sclera.
I generally use the “prior RK” function of the postrefractive surgery IOL calculator provided on the ASCRS's website by Warren Hill, MD; Li Wang, MD, PhD; and Douglas Koch, MD. I usually bump the power up by around a diopter, because I find the calculator often underpowers the implant. I would rather that a post-RK eye end up a little myopic than a little hyperopic. It is easy to touch up myopic results with PRK.
I would also aggressively polish the anterior capsule at the time of surgery so that I could perform an IOL exchange in the event of a large error in power.
Under no circumstance would I implant a multifocal IOL. I would also hesitate to implant a toric IOL unless the cylinder was very high.
Preoperative counseling regarding the difficulty of performing IOL power calculations in the postrefractive surgery setting is key to avoiding an unhappy patient.
GEORGE O. WARING IV, MD
IOL patients with prior RK have become an appreciable part of my practice. This patient should be educated about her condition and the considerations surrounding IOL surgery after RK. It is important to set appropriate expectations and outline them in an informed consent. Specifically, she should understand that delayed visual recovery is the rule with IOL surgery after RK, that the IOL procedure will not fix any preoperative fluctuations in vision, and that she has a higher likelihood of needing a laser or IOL enhancement, an IOL exchange, or contact lenses/glasses after surgery than someone without prior RK.
Because this patient appears to have undergone 16-cut RK and to have a history of fluctuating vision, I would obtain a diurnal preoperative manifest refraction, topography, tomography, and optical biometry. For 16-cut RK, I target a small amount of postoperative myopia, typically -0.75 to -1.00 D, and use both the ASCRS website for postrefractive surgery eyes and the post-RK calculation in the Holladay IOL Consultant (Holladay Consulting). I also pay attention to placido central keratometry readings. Because this patient's UCVA improves significantly with astigmatic correction, I would take a weighted mean of diurnal measurements to determine the magnitude and orientation of astigmatic correction. In this case, I would suggest a toric IOL, assuming the topography and tomography are somewhat regular, given the improvement in UCVA with astigmatic correction.
A scleral tunnel would be my preferred technique here to minimize the risk of opening RK incisions. I would leave the lasso suture, which will stabilize the RK incisions from both mechanical and optical standpoints. Nonetheless, I would have additional sutures readily available in the event that an RK incision opened. In the post-RK setting, I also perform intraoperative aberrometry, and I use the intraoperative data to guide my preoperative calculations, again erring toward a myopic target.
Postoperatively, I would expect a mildly hyperopic result, and I would reassure the patient that this was a good sign and that refractive stabilization will take time. I would also remind her that visual fluctuations might persist.
ROBERT J. WEINSTOCK, MD
Post-RK cataract patients can pose a real challenge. Refractive instability from a weak cornea, irregular astigmatism from the incisions, and corneal multifocality across the visual axis—depending on pupillary size—are a few of the issues commonly encountered.
In this particular case, I feel the beautifully placed encircling suture actually makes things easier, and I would leave it alone if it looked good. For IOL power calculations, I would acquire multiple keratometry readings with varying diagnostic devices such as the Galilei Dual Scheimpflug Analyzer (Ziemer Ophthalmic Systems), Cassini (i-Optics), Lenstar LS900 (Haag-Streit), and a manual keratometer. I would also look at photopic and mesopic pupil overlays on topography to best guess the flattest keratometry readings in the pupillary/visual axis. I would use aphakic intraoperative aberrometry as an additional data point prior to final lens power selection, and I always target a little minus for these patients to account for progressive corneal flattening. I am less trusting of pseudophakic readings with the ORA System (Alcon WaveTec) in RK patients.
Clearly, this patient needs to be counseled about the difficulties of selecting the accurate IOL power and educated that she may continue to experience diurnal fluctuations in vision from the weakened corneal architecture. I also tell all RK patients that their vision may not clear or stabilize for 2 or 3 weeks after surgery. In my experience, many of these patients refract to +2.00 D a few days postoperatively but then experience a drift toward plano after 2 weeks. In addition, I explain that they may require glasses or contact lenses in the future, and I discuss the rare need for an IOL exchange within the first month for a big refractive surprise and the possibility of PRK several months out if warranted. I would use a bimanual microincisional cataract surgery technique with microwounds placed at the limbus between the RK incisions. Using a wound-assisted technique through the 1.6-mm incision, I would insert an Akreos MICS lens (Bausch + Lomb) because of its aspheric 0.00 D aberration design and ease of cutting if removal and exchange were needed. n
Section Editor Alan N. Carlson, MD
• professor of ophthalmology and chief, corneal and refractive surgery, Duke University Eye Center, Durham, North Carolina
• (919) 684-5769; carls009@mc.duke.edu
Section Editor Stephen Coleman, MD
• director of Coleman Vision, Albuquerque, New Mexico
Section Editor Karl G. Stonecipher, MD
• director of refractive surgery, TLC in Greensboro, North Carolina
Uday Devgan, MD
• private practice, Devgan Eye Surgery, Los Angeles
• chief of ophthalmology, Olive View UCLA Medical Center
• clinical professor of ophthalmology, Jules Stein Eye Institute, UCLA School of Medicine
• (800) 377-1969; devgan@gmail.com; Twitter @devgan
Kevin M. Miller, MD
• Kolokotrones chair in ophthalmology, David Geffen School of Medicine at UCLA, Jules Stein Eye Institute, Los Angeles
• (310) 206-9951; kmiller@ucla.edu
George O. Waring IV, MD
• director of refractive surgery and an assistant professor of ophthalmology, Storm Eye Institute, Medical University of South Carolina
• medical director, Magill Vision Center, Mt. Pleasant, South Carolina
• waringg@musc.edu; Twitter @georgewaring
• financial disclosure: none acknowledged
Robert J. Weinstock, MD
• director of cataract and refractive surgery, Eye Institute of West Florida, Largo, Florida
• rjweinstock@yahoo.com; Twitter @EyeInstituteWFl
• financial disclosure: consultant to Abbott Medical Optics, Alcon, and Bausch + Lomb


