Dry eye disease (DED) is one of the most common complications of refractive surgery, and it can have devastating consequences in some patients. A stable, healthy tear film is essential to high-quality visual function. The ideal method of preventing DED after refractive surgery is a meticulous perioperative management plan as opposed to postoperative management alone. The former begins with the prudent screening of patients combined with aggressive evaluation and treatment of the condition preoperatively.
IDENTIFYING THOSE AT RISK
Identifying patients at risk of DED after refractive surgery is essential to minimizing complications and optimizing surgical outcomes. Female gender, age, diabetes, and smoking are common predisposing factors to postoperative DED. Contact lens wear is another important factor, and a history of contact lens intolerance should raise a red flag.
The history should include careful questioning about DED symptoms. I like to use either the Standard Patient Evaluation Eye Dryness (SPEED) or the Ocular Surface Disease Index (OSDI) questionnaire, which are both validated, to screen patients. Symptoms to inquire about include gritty, irritated, dry, and/or tired eyes and fluctuations in vision, especially when associated with blinking.
ADVANCES IN EVALUATION AND DIAGNOSIS: POINT-OF-CARE TESTING
Refractive surgery candidates should undergo a detailed evaluation of their ocular surface. There are several traditional but valuable ways of assessing the surface, including tear breakup time, corneal staining, and evaluating patients for the presence of meibomian gland dysfunction (MGD; Figure 1). Current options for evaluating and diagnosing DED have changed dramatically over the past several years with point-of-care testing. Although there is no single diagnostic test for DED, there is increasing evidence that evaluating tear osmolarity can provide crucial information about disease severity and therapeutic response.
InflammaDry (Rapid Pathogen Screening) is a newer test that can detect matrix metalloproteinase 9 (MMP-9), an inflammatory marker that may provide evidence of early ocular surface disease. The leading cause of DED, MGD is a progressive disease that, if not treated, can lead to glandular atrophy and loss of function.
Recently introduced, LipiView II (TearScience) provides high-definition images of the meibomian glands to reveal if they are dilated, truncated, or atrophied (Figures 2 and 3). This device also measures the absolute thickness of the lipid layer of the tear film and identifies the number of partial blinks.
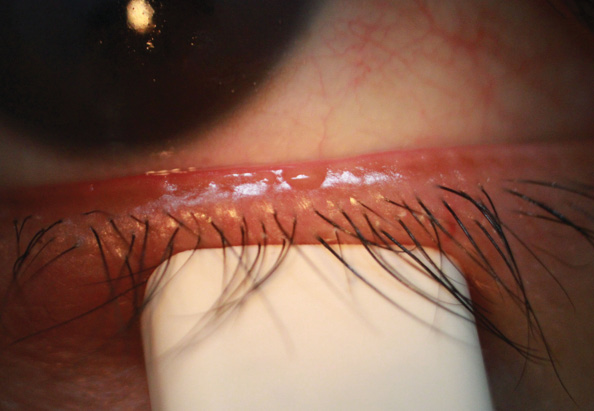
Figure 1. Eighty-six percent of all DED is attributed to MGD. The latter may result in an unstable tear film, ocular surface disease, and inflammation as well as bring about symptoms of irritation, burning, and ocular fatigue.
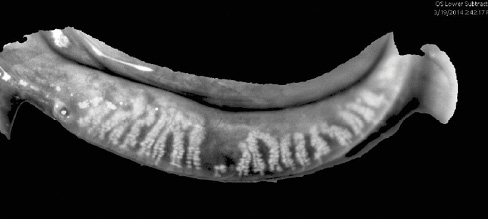
Figure 2. Dilation and truncation of the meibomian glands. MGD is a chronic, prevalent, and progressive disease. Visualizing gland structures makes the detection and management of MGD easier.
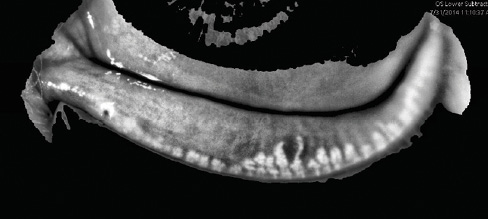
Figure 3. Chronic inflammation and blockage cause dropout and atrophy, resulting in irreversible damage to the meibomian glands. Treating patients before damage occurs is preferable to waiting until the glands have atrophied and ceased to function.
Severe and refractory DED can develop after refractive surgery in patients with Sjögren syndrome; one out of 10 patients with DED are thought to have this autoimmune disease. An advanced diagnostic panel for the early detection of this condition, Sjö (Nicox), offers high specificity and sensitivity. Diagnosing Sjögren syndrome prior to laser vision correction allows eye care practitioners to be proactive instead of reactive.
AGGRESSIVE PREOPERATIVE TREATMENT NEEDED
PRK and LASIK may exacerbate pre-existing DED or trigger a previously asymptomatic dry eye. Preoperative recognition of dry eyes and conscientious attention to and treatment of the ocular surface in the perioperative period can promote more accurate visual outcomes and enhance patients' satisfaction. To reduce the risk of compromised visual acuity after keratorefractive procedures, I implement an aggressive DED treatment plan.
The primary goal of treating moderate to advanced DED is to reduce inflammation of the ocular surface. Topical cyclosporine pre- and postoperatively can be extremely helpful for increasing natural tear production and treating an unstable, hyperosmolar tear film.1 Using a corticosteroid preparation that is gentle on the ocular surface as well as safe and effective can rapidly reduce inflammation and work synergistically with cyclosporine. A majority of patients with DED have an evaporative component.2
I routinely start refractive surgery patients on omega-3 nutritional supplements, which have been shown to improve tear osmolarity, corneal staining, and Ocular Surface Disease Index and omega index levels (A.E., E. Donnenfeld, MD, et al, unpublished data, 2015). My favorite formulation comes from PRN Physician Recommended Nutraceuticals; it is very pure (ie, toxins and mercury are removed), and the triglyceride form is more absorbable than the less expensive ethyl ester forms of omega-3 fatty acid supplements. There is also evidence that omega-3 may speed epithelial healing and visual recovery after PRK3 and may help with nerve regeneration.4
I find topical azithromycin helpful in patients with mild to moderate anterior or posterior blepharitis (an off-label use). The LipiFlow Thermal Pulsation System (TearScience) has been revolutionary in my dry eye practice. It unblocks meibomian glands via heat and pulsatile pressure. LipiFlow is the only FDA-approved treatment for evaporative DED, and it also disrupts disease progression. Once the integrity of the tear film is re-established, my next goal is to enable patients' eyes to retain tears for longer through punctal occlusion. If this modality is employed prematurely, I find that patients tend to be more uncomfortable, because they still have inflammatory mediators in the tear film.
CONCLUSION
DED is a recognized but often preventable complication in patients undergoing refractive surgery. The disease decreases surgical predictability and can adversely affect visual outcomes. Identifying patients at risk of DED and optimizing the ocular surface perioperatively can reduce problems. n
1. Sullivan BD, Crews LA, Sönmez B, et al. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea. 2012;31(9):1000-1008.
2. Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-478.
3. Ong NH, Purcell TL, Roch-Levecq AC, et al. Epithelial healing and visual outcomes of patients using omega-3 oral nutritional supplements before and after photorefractive keratectomy: a pilot study. Cornea. 2013;32(6):761-765.
4. He J, Bazan HE. Omega-3 fatty acids in dry eye and corneal nerve regeneration after refractive surgery. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4-6):319-325.
Alice Epitropoulos, MD
• founder/owner, Ophthalmic Surgeons & Consultants of Ohio—The Eye Care Center of Columbus
• clinical assistant professor, The Ohio State University
• (614) 221-7464; aepitrop@columbus.rr.com
• financial disclosure: consultant to and on the speakers' bureaus of Allergan, Bausch + Lomb, Nicox, and Shire; contracted researcher for PRN Physician Recommended Nutraceuticals and TearLab; preceptor for TearScience


