Sticking With Brand Names
By Quentin B. Allen, MD

In the wake of the COVID-19 pandemic, there have been many changes in our ophthalmology practice. To maintain some sense of normality amid so much change, however, we have elected to keep our cataract surgery drop regimen the same as before. For me, this means sticking with two branded drugs to ensure the best quality outcomes.
DROP PREFERENCES
NSAID. I use bromfenac ophthalmic solution 0.075% (BromSite, Sun Ophthalmics). I ask patients to begin using it q.d. at bedtime, 3 days before surgery, for prevention of pain, miosis, and cystoid macular edema (CME). Formulated with the topical polymeric drug delivery system DuraSite (InSite Vision), this NSAID has excellent retention and residence time, increasing its penetration to target tissues. Patients continue its use for 6 weeks postoperative at a minimum.
Antibiotic. In addition to injecting intracameral moxifloxacin at the time of surgery, I ask patients to start using a generic fluoroquinolone (moxifloxacin or ofloxacin) drop t.i.d. for 3 days before surgery and to continue it for 7 days postoperatively.
Steroid. I have found great success with loteprednol etabonate ophthalmic suspension 1% (Inveltys, Kala Pharmaceuticals). This is a surface-modified loteprednol nanoparticle with excellent penetration and a favorable side effect profile (Figure). Patients start the drug b.i.d. immediately after surgery and continue for 6 weeks with no taper.
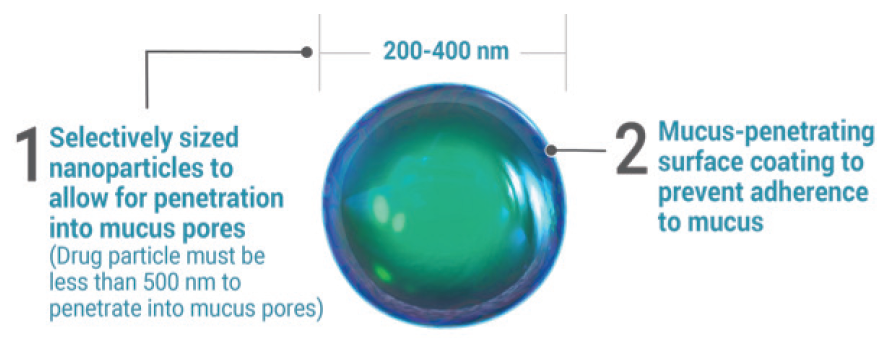
Figure. The size of the nanoparticles in Inveltys help to penetrate the mucus pores.
Courtesy of Kala Pharmaceuticals
IN PURSUIT OF PREMIUM OUTCOMES
I rely on the consistency and high tolerability of the brand name steroid and NSAID eye drops to rapidly quench postoperative inflammation and provide premium outcomes. In Florida, as in most parts of the country, cost and pharmacy generic substitution are major issues. In order to ensure that my patients actually obtain the medications I prescribe, I have found the greatest consistency by using a specialty mail service pharmacy (Benzer Pharmacy) to standardize patients’ cost and experience and to prevent generic substitution.
Enhanced Penetration, Simplified Dosing
By Ivan Mac, MD, MBA

There are many options for steroid therapy after cataract surgery. I prefer the use of a relatively new formulation, loteprednol etabonate 1%, which was introduced to the market in 2019. The formulation uses selectively sized nanoparticles with a mucus-penetrating surface coating to enable the drug to move through the tear film’s mucous layer and to penetrate ocular tissue better than traditional suspensions (Figure).
I have been using this formulation of loteprednol for nearly all my cataract patients for about a year. The regimen starts 2 days before surgery and continues for 2 weeks after surgery, along with an NSAID and an antibiotic. I’ve been satisfied with the results for three primary reasons: efficacy, safety, and compliance.
EFFICACY AND SAFETY
Like other corticosteroids we prescribe after cataract surgery, this drug controls inflammation and pain. Further, its mucus-penetrating particle technology works to penetrate into the anterior chamber so that a high level of loteprednol is delivered inside the eye.
My patients’ eyes are quiet on postoperative day 1 with no corneal edema, and I rarely see signs of inflammation throughout recovery. I also like that the drug’s active ingredient is loteprednol, a steroid that has long been shown to be safe, well tolerated, and quickly metabolized.1
COMPLIANCE
For postoperative cataract surgery patients, I always want to use drugs that deliver safe and effective results, preferably using as few daily drops as possible to minimize compliance problems that are inherent to complex multidrop regimens. Other steroid drops are usually used t.i.d. or q.i.d., but Inveltys labeling specifies b.i.d. dosing after surgery.2
Before this formulation became available, I had tried using other steroids b.i.d. off-label to improve compliance, but some patients experienced elevated IOP. I’m seeing improved compliance with the b.i.d. dosing of Inveltys with no significant elevation in IOP.
CONCLUSION
The transition to using this drug has been smooth. It works well with a safety profile that’s reassuring, and patients report that they find it easy to use.
1. Comstock TL, Sheppard JD. Loteprednol etabonate for inflammatory conditions of the anterior segment of the eye: twenty years of clinical experience with a retrometabolically designed corticosteroid. Expert Opin Pharmacother. 2018 Mar;19(4):337-353.
2. Inveltys [package insert]. Watertown, MA; Kala Pharmaceuticals. April 2020.
Simplifying Routines Benefits Patients and the Practice
By Shachar Tauber, MD

In late 2018, the surgeons at Mercy Clinic made a commitment to wholesale changes in our perioperative drop regimen for anterior segment surgery, including cataract, cornea, and laser refractive surgery procedures. This change was undertaken because we had been informed by our technicians and nurses that our patients were struggling with issues regarding copays, deductibles, overall cost, and availability, as well as drop compliance due to complexity and confusion.
Further, tales from our call center relayed high patient dissatisfaction with our multidrop branded and generic drop regimens. Staff members were spending hours away from patient care, acting as commodity brokers to help patients access their proper perioperative medications.
We soon found the compounding pharmacy OSRX and its Omni formulations, which combine everything we need into a single drop. The one we chose to employ includes an antibiotic (gatifloxacin 0.5%), a steroid (prednisolone phosphate 1.0%), and an NSAID (bromfenac 0.075%). There is also an option to delete the NSAID component for our LASIK and PRK patients. The drops can be ordered at the time of surgery consultation and are shipped to the patient’s home before the surgery date. The cost is modest—certainly less than any drug coupon programs available.
We sent our pharmacists to visit OSRX’s 503A compounding pharmacy in Montana. (Editor’s note: For more on compounding pharmacies, see “Compounded Therapeutics: Seven Situations When a Closer Look at Compounding Pharmacy Options is Warranted”) They returned feeling that quality, safety, and redundancies were excellent at the facility.
By Christmas 2018, all our patients were receiving their perioperative drops at home to be used t.i.d. for 3 days before surgery and then for 3 weeks postoperatively. Patient satisfaction was markedly improved, and patient call-backs returned to easily manageable numbers. Most important, our staff members loved their Christmas present of being able to spend more time providing direct patient care. They were most grateful to be freed from the frustration of dealing with medication costs and availability issues.
HELPFUL UPDATES
OSRX now has a HIPAA-compliant, secure prescriber portal, which allows prescriptions to be submitted, managed, and tracked online. This feature has increased our level of transparency and control and further reduced patient callbacks.
Additionally, in a bid to help practices reopen effectively with proper social distancing, the company is offering a dilation drop (cyclopentolate 1%/phenylephrine 2.5%) and brimonidine 0.2%. The cyclopentolate-phenylephrine allows patients to dilate themselves before arriving for surgery, expediting the preoperative process and reducing wait times.
The brimonidine can be preemptively dosed postoperatively to mitigate the potential for IOP spikes. Taking this preventive measure, in turn, allows us to perform virtual postoperative day 1 examinations and reduce the number of patient follow-up visits. The cost of these medications is nominal (cyclopentolate-phenylephrine, $15 per bottle; brimonidine, $11 per bottle), and they can be shipped directly to your practice or to patients at no added cost.
These changes in our drop regimens are all significant wins, especially now in the post–COVID-19 surgical world.
Modifying the Postop Regimen Can Improve Compliance
By Nandini Venkateswaran, MD; and Terry Kim, MD


In our clinical practice, we do not routinely pretreat patients before cataract surgery. In specific circumstances, such as in patients with a history of uveitis or a high risk for CME, patients are given topical and oral steroids or topical NSAID drops preoperatively for 3 to 7 days to reduce the risk of postsurgical inflammation.
Intraoperatively, in both manual and laser cataract surgery, all patients receive phenylephrine and ketorolac 1%/0.3% intraocular solution (Omidria, Omeros) to maximize pupillary dilation and reduce postoperative pain.
POSTOPERATIVE CHOICES
Postoperatively, we implement a combination of drops to treat inflammation and prevent infection and CME. Our current regimen includes the generic antibiotic ofloxacin 0.3% b.i.d. for 1 week; the steroid loteprednol etabonate ophthalmic suspension 1%, with its Ampplify technology that enhances ocular surface penetration, b.i.d. for 2 weeks; and the NSAID bromfenac 0.075% (BromSite, Sun Pharmaceuticals) b.i.d. for 4 weeks. The frequency of the steroid drops can be increased if corneal edema or anterior chamber inflammation is seen postoperatively.
These medications are obtained easily by most patients through their local North Carolina pharmacy. However, for patients whose insurance plans do not cover these medications, substitutions are made with generic formulations of prednisolone acetate 1% and ketorolac 0.5% t.i.d. to reduce costs and improve compliance.
STEROID INSERT
Over the past several months, we have routinely used the preservative-free intracanalicular dexamethasone ophthalmic insert 0.4% (Dextenza, Ocular Therapeutix) to replace the topical corticosteroid drop postoperatively. At the conclusion of cataract surgery, we insert this steroid rod into the lower eyelid canalicular system after dilation of the lower eyelid punctum in less than 1 minute. Rarely does a patient’s punctal or canalicular anatomy preclude insertion of this device, and patients greatly appreciate having to use one less postoperative topical medication. (Editor’s note: For more on this and other drug delivery platforms, see “New Approaches to Drug Delivery.”)
Currently, we are primarily implanting this device in our patients with Medicare Part B insurance plans to ensure coverage. With today’s challenges of patient compliance, medication cost, and patient callbacks (what we call the 3 Cs of Topical Medication Issues), this novel drug delivery platform has been welcomed in our practice and has also helped to minimize eye and face touching in the COVID-19 era.

Doing Away With Drops
By Denise M. Visco, MD, MBA

Before the COVID-19 pandemic, our practice was already moving toward minimizing perioperative drop regimens for cataract surgery with the goal of improving patient compliance. Concerns surrounding the pandemic have pushed us to eliminate drops altogether. Let me explain how we got here.
Regarding antibiotic use with cataract surgery, before the pandemic our surgeons had concerns about replacing the branded topical formulation we had been using with intracameral compounded moxifloxacin only. Surgeons were using intracameral moxifloxacin but still prescribing topical antibiotics perioperatively.
Antiinflammatory agents were a different story. When phenylephrine and ketorolac intraocular solution 1%/0.3% became available at our ambulatory surgery center several years ago, we dropped topical steroids from our cataract protocol. This made life much easier. No complex tapering schedules for patients, and no chasing down the appropriate formulary steroid product.
Thus, even before the pandemic, our cataract surgery patients used only topical NSAID and antibiotic perioperatively. Bromfenac ophthalmic solution 0.075% and besifloxacin ophthalmic suspension 0.6% (Besivance, Bausch + Lomb) were each used b.i.d. for 2 days preoperatively; besifloxacin was continued for 1 week and bromfenac for 4 weeks postoperatively.
We did a retrospective study of our patients before and after this change and found that patients receiving intracameral phenylephrine-ketorolac had a lower incidence of CME, pain, and breakthrough iritis postoperatively than those receiving the topical steroid.1 Not only did our new protocol maintain our positive patient experience, we improved it!

With the advent of two sustained delivery steroid products—the intracameral dexamethasone intraocular suspension 9% (Dexycu, EyePoint Pharmaceuticals) and the intracanalicular dexamethasone ophthalmic insert 0.4 mg—we began considering a return to steroids for postoperative inflammation control if we could forfeit the topical NSAIDs.
The primary advantage of this change, obviously, would be eliminating another product that depends on patient compliance. An additional advantage, however, would be decreasing the burden of patient prescriptions on our practice workload. For reference, when we discontinued topical steroids, we cut this workload by a third. We could potentially decrease the burden by an additional third, leaving only the antibiotic. We began using the intracanalicular dexamethasone ophthalmic insert for patients with insurance coverage.
Then COVID-19 arrived. Patient safety issues now include limiting patient visits to the office and to pharmacies. Full and hybrid telemedicine examinations are a new normal for our practice. The administrative burden of office visit maintenance and documentation has increased dramatically. Ironically, we must do less with more. The reservations we were holding onto no longer seemed relevant. Consequently, we’ve embraced a drop-free regimen because this approach philosophically hits all the new post–COVID-19 benchmarks of patient care.
We have discontinued topical antibiotics and topical NSAIDs; we prescribe bromfenac b.i.d. only if patients cannot have the intracanalicular dexamethasone insert or are at high risk for CME postoperatively.
Our current patient-compliance-independent perioperative cataract surgery protocol is outlined in the accompanying sidebar above.
1. Visco DM, Bedi R. Effect of intracameral phenylephrine/ketorolac 1.0%/0.3% on postoperative cystoid macular edema, iritis, pain, and photophobia after cataract surgery. J Cataract Refract Surg. 2020;46(6):867-872.
It Only Takes One Drop, and No Steroid
By Keith A. Walter, MD, FACS

For the past 6 years, I’ve been prescribing just one drop, bromfenac, once a day starting 2 days before cataract surgery and then continued for 28 days afterward. I choose this regimen for all patients, including those with dense lenses, dark irises, and diabetes, and in patients who require a pupil expansion device. I do not prescribe a steroid unless the patient has a history of uveitis or the eye has retained lens material; either condition can induce a lot of T cell–type inflammation and immune responses. I haven’t routinely used a steroid in more than 10 years.
Even before transitioning to this one-drop regimen, I had prescribed only two drops postoperatively, an NSAID and an antibiotic. To further reduce the burden on patients, about 6 years ago I decided to switch from prescribing an antibiotic drop after cataract surgery to administering moxifloxacin intracamerally during surgery.
NO MORE STEROID-TAPERING SCHEDULES
A once-a-day drop therapy with no steroid is easier for patients because it eliminates the need for them to follow a steroid-tapering schedule. In the past, patients had trouble following different instructions for two or three drops, with two or three different copays and pharmacy callbacks. Compliance was low, and many times this affected postoperative outcomes.
Bromfenac is an excellent topical NSAID with a good safety profile, which helps me feel comfortable enough to eliminate the use of a steroid. For surgeons who are apprehensive about not prescribing a steroid, I would suggest considering the use of phenylephrine and ketorolac intraocular solution 1%/0.3%, which we have found helps to reduce the incidence of postoperative CME to 0.40%.1 This treatment is added to the irrigation solution to prevent intraoperative miosis and reduce postoperative ocular pain.
If you think about it, ophthalmologists are the only surgeons to put a steroid on an operative wound. Orthopedic surgeons don’t do it. Neurosurgeons don’t do it. They don’t even prescribe oral steroids or give IV steroids because steroids delay wound healing and increase the risk of infection. Plus, in our specialty, they increase the risk for raised IOP.
CONCLUSION
In my opinion, prescribing a good NSAID like bromfenac or using intracameral ketorolac precludes the need for steroid use after cataract surgery, even if we see a little cell and flare. I have been using this regimen for the past 6 years, and before that a two-drop, no-steroid regimen for 4 years, with excellent results. Patient compliance has increased substantially, and postoperative results have improved.
1. Walter K, Kauffman L, Hess J. Rate of pseudophakic cystoid macular edema using intraoperative and topical nonsteroidal antiinflammatory drugs alone without steroids. J Cataract Refract Surg. 2020;46(3):350-354.
Compounded Meds Are the Way to Go
By Gary Wörtz, MD

In my opinion, the biggest factor in determining the best pre- and postoperative medication regimen for cataract surgery is simplicity. Faced with multiple medications in different bottles requiring different administration schedules, patients can become confused. This can lead to patients’ lack of adherence to designated regimens, whether intentional or not.
For this reason, I prefer compounded drops. In particular, the fixed combination of the steroid prednisolone acetate 1.0%, the antibiotic gatifloxacin 0.5%, and the NSAID bromfenac 0.075% is my go-to drop. Following cataract surgery, patients are prescribed Omni q.i.d. for the first 2 weeks postoperatively and then b.i.d. for the following 2 weeks. With this regimen, in more than 5,000 consecutive cataract surgeries, I have seen no increase in inflammation, including macular edema, and no incidence of endophthalmitis.
Other companies offer similar compounding pharmaceuticals. Surgeons should review each product and determine which combination is best for them. The use of compounded drops simplifies things for patients, who no longer must distinguish between tiny bottles and attempt to comply with a complex drop regimen. It also simplifies things for the practice staff.
Some steroid products have been granted pass-through status. Although these long-acting depots represent another novel approach to improving patient compliance, I feel that navigating the bureaucratic process necessary to take advantage of these products is a hassle and too time-consuming, especially when good alternatives exist.