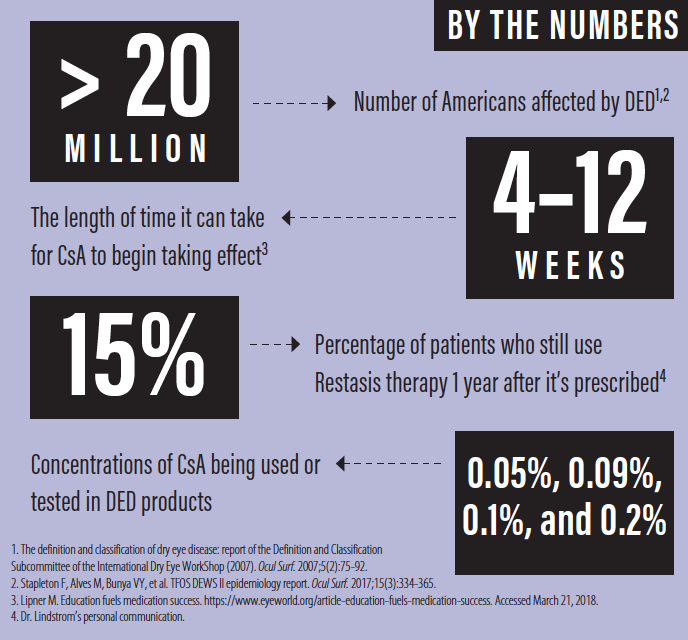
Globally, the prevalence of dry eye disease (DED) ranges from 5% to 50% of the population, with more than 20 million Americans affected.1,2 Although not usually sight-threatening, DED has a negative impact on patient quality of life and on the success of refractive surgery and contact lens wear.
Historically, the multifaceted nature of DED and the frequent lack of correlation between its signs and symptoms have made the condition difficult to treat. For years, the only FDA-approved topical treatment was »Restasis (cyclosporine ophthalmic emulsion 0.05%, Allergan). Although this drug is going off-patent, cyclosporine A (CsA) remains relevant for the management of patients with DED.
CONVENIENCE, COMPLIANCE, AND COST
Even before the introduction of Restasis in 2002, cornea specialists sometimes ordered compounded CsA. The drug was also compounded for use as a steroid-sparing agent to reduce the risk of rejection in high-risk keratoplasty procedures.
CsA is an immunosuppressant that inhibits T-cell–mediated inflammation and cytokines that have been implicated in the pathogenesis of DED. The drug has been shown to stimulate tear production3 and increase goblet cell density.4
Although a number of studies have shown CsA 0.05% to have beneficial effects on Schirmer test scores and ocular staining, evidence in the literature of symptomatic relief has been less consistent.5 Many clinicians, myself included, are confident that Restasis has helped our patients, but compliance with prescribed medical therapy is a well-known challenge. It can take 4 to 12 weeks for CsA to begin taking effect, and during this period, stinging on instillation and/or spectacle blur can discourage patients from using the drops.6
Other physicians and I have often addressed this challenge by pairing Restasis with a topical steroid to bring more immediate relief. Nevertheless, the data suggest that only 15% of patients who start Restasis therapy will still be using it 1 year after it was prescribed (personal communication). The drop-off may be attributable to lack of efficacy for some patients, the cost of the medication, or simply the difficulties of long-term adherence and persistence with any topical medication for chronic disease.
As Restasis goes off-patent, it is natural to assume that generic versions will become available that will reduce the cost of the medication. Generic drug manufacturers, however, will find that one of the challenges in delivering CsA to the ocular surface is the drug’s poor solubility, which means it is difficult to devise a solution with a comfortable diluent in which the active ingredient will stay properly mixed. Generic formulations are also not likely to be available in the multidose, nonpreserved form that many patients and doctors prefer.
HIGHER-CONCENTRATION FORMULATIONS
Several companies have introduced eye drops with higher concentrations of CsA than Restasis. CsA 0.1% (Ikervis, Santen) was approved for daily use in Europe in 2015. Likewise, Sun Pharma has been testing Seciera, with a concentration that is also nearly twice that of Restasis (0.09% vs 0.05%). In randomized, double-masked, vehicle-controlled, phase 3 studies, Seciera has been shown to be noninferior to Restasis, indicating that the higher concentration works at least as well as the 0.05% concentration in Restasis;7 what is not clear, however, is whether the higher concentration is more effective. Novaliq recently completed a phase 2 clinical trial of CyclASol (CsA 0.05% and 0.1% dissolved in EyeSol, the company’s novel semifluorinated alkane delivery technology).8 Results were promising, and phase 3 studies of CyclASol are expected to begin soon. It is to be hoped that these higher concentrations may be slightly more effective than Restasis. Certainly, they should be safe, given that 0.1% and even 0.2% CsA products are currently in use in humans and dogs and that compounded CsA of up to 2% has been used in the past without incident.9
CSA IN CHONDROITIN SULFATE
I have been involved in the development of »Klarity-C (Imprimis Pharmaceuticals), CsA 0.1% in a chondroitin sulfate emulsion. These drops will be available as a customized compounded agent, first under the 503A program and later the 503B program (see Pharmaceutical Channels in the United States below).
PHARMACEUTICAL CHANNELS IN THE UNITED STATES
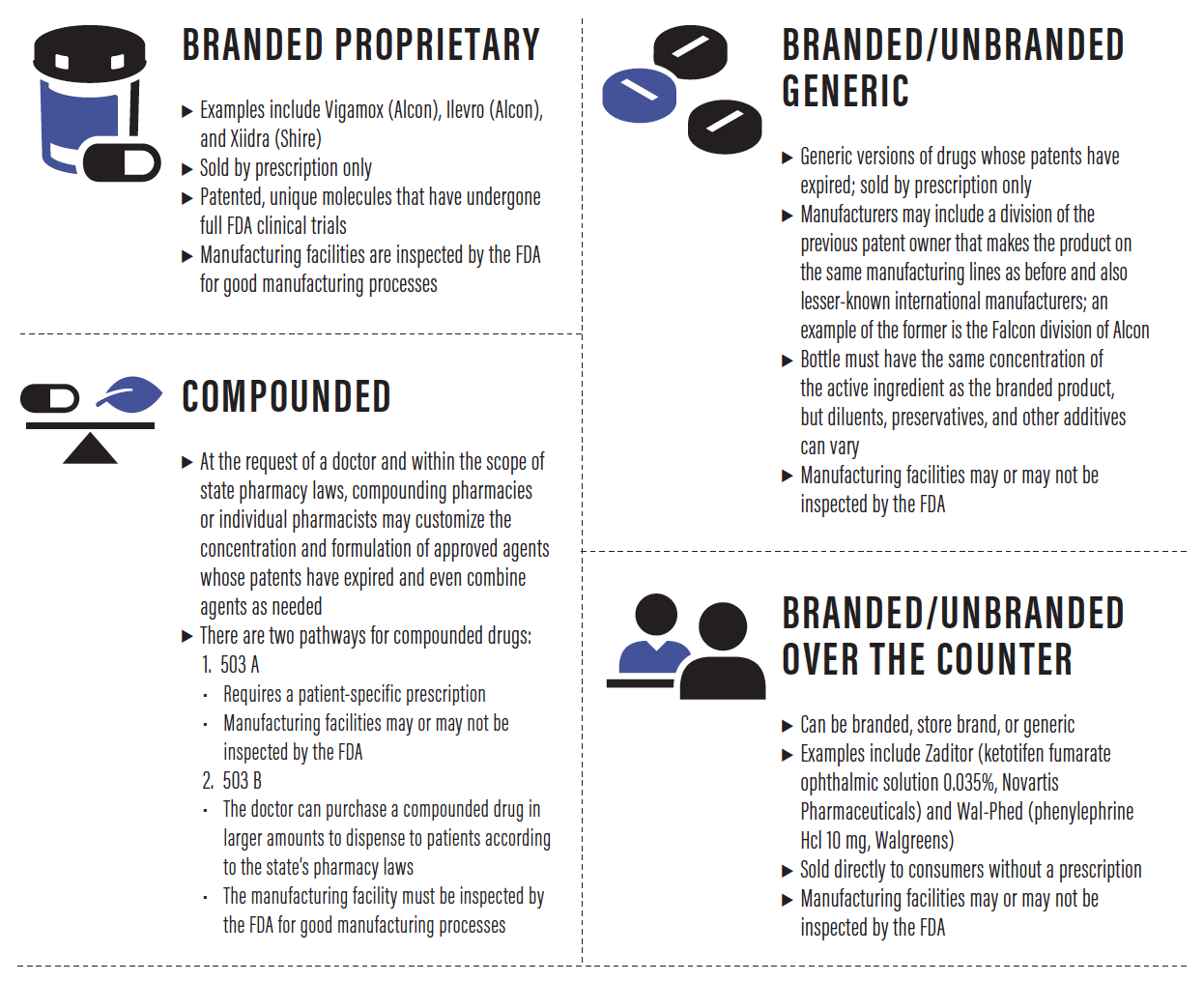
Until relatively recently, all eye drops were compounded. Doctors would ask their local pharmacist to mix a particular concentration of a desired active ingredient in normal saline, for example. In the early 1950s, the first ophthalmic companies, Allergan and Alcon, began mass producing some popular compounded eye drops and launched the first branded proprietary eye drops. Today, there are four channels for the sale and distribution of eye drops in the United States.
Ophthalmologists are familiar with chondroitin sulfate as the key ingredient in Optisol GS (manufactured by Bausch + Lomb, distributed by various companies), the solution used to preserve about 80% of the corneal tissue used in transplant procedures. Chondroitin sulfate is also present in several of the OVDs used to protect the cornea during cataract surgery.
Chondroitin sulfate has several properties that make it ideal for a DED drop.10 First, it is an excellent lubricant. Second, as a free radical scavenger antioxidant, chondroitin sulfate has antiinflammatory properties of its own, even before adding CsA. Third, chondroitin sulfate is also a cell membrane stabilizer, which enhances healing and reduces cell death and apoptosis. Fourth and finally, this compound has oncotic pressure capabilities, which means that it reduces corneal—and even stromal—edema. This last characteristic is what has made chondroitin sulfate so useful for preserving donor corneal tissue, but it is also easy to imagine the value of reducing edema in vivo in a cornea stressed from contact lens wear, severe DED, or surgical insult.
Klarity-C also includes dextran, glycerol, and hydroxypropylmethylcellulose, all of which are common lubricating components with long track records in ophthalmology. However, I believe the chondroitin sulfate is what makes these eye drops unique. They are not preserved and are dosed twice daily, although physicians may prescribe them for more or less frequent use. Klarity-C could be further customized by compounding it with a complementary agent such as dexamethasone or testosterone to address ocular inflammation or treat androgen-deficiency DED.
EXTENDED RELEASE
New methods of extended-release drug delivery will likely play an important role in CsA’s future. Work is well under way to deliver CsA via a drug-eluting ring insert (device formerly called Helios and developed by ForSight Vision,5 now owned by Allergan). The ring, which rests on the conjunctiva under the upper and lower eyelids, is in phase 2 clinical trials for delivering an IOP-lowering agent for the treatment of glaucoma, but the same technology could also deliver antiinflammatory or other ocular surface therapies.
Punctal plugs can help patients maintain a greater tear volume, and the devices present a compelling platform for delivering CsA to the target tissues of the ocular surface. Both Ocular Therapeutix and Mati Therapeutics are investigating the extended release of CsA with their respective punctal plug technologies.
CONCLUSION
CsA is a time-tested, effective drug that achieves the desired downregulation of T-cell–mediated inflammation in DED. Going forward, I expect to see the drug available in branded, generic, and compounded formulations as well as perhaps an extended-release form. All of the activity surrounding new products suggests to me that, even as drugs such as lifitegrast ophthalmic solution 5% (Xiidra, Shire) and others enter the DED treatment arena, there is still plenty of opportunity for innovation with CsA.
1. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):75-92.
2. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334-365.
3. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107(4):631-639.
4. Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120(3):330-337.
5. Rhee MK, Mah FS. Clinical utility of cyclosporine (CsA) ophthalmic emulsion 0.05% for symptomatic relief in people with chronic dry eye: a review of the literature. Clin Ophthalmol. 2017;11:1157-1166.
6. Lipner M. Education fuels medication success. https://www.eyeworld.org/article-education-fuels-medication-success. Accessed March 21, 2018.
7. Sun Pharma announces positive topline results of confirmatory phase 3 clinical trial for Seciera for treatment of dry eye. https://www.prnewswire.com/news-releases/sun-pharma-announces-positive-topline-results-of-confirmatory-phase-3-clinical-trial-for-seciera-for-treatment-of-dry-eye-609638655.html. Accessed March 21, 2018.
8. Torkildsen GL, Moreira HR, Wirta DL, et al. A clinical phase 2 study to assess efficacy, safety, and tolerability of CyclASol for treatment of dry eye disease. Poster presentation PO059 at the: 2017 American Academy of Ophthalmology Meeting; New Orleans; November 11-14, 2017.
9. Lallemand F, Shmitt M, Bourges JL, et al. Cyclosporine A delivery to the eye: a comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm. 2017;117:14-28.
10. Lindstrom RL, Kaufamn HE, Skelnik DL, et al. Optisol corneal storage medium. Am J Ophthalmol. 1992;114(3):345-356.