
The current standard of care in cataract surgery is phacoemulsification in developed countries and manual small-incision cataract surgery (MSICS) in developing countries. The first femtosecond laser cataract surgery, performed in 2008, marked the beginning of a revolutionary advance in this field.1
But the first use of lasers in the cataract surgical procedure predates the femtosecond laser. In 1993, the first procedure for the removal of a nuclear sclerotic cataract using high-powered Nd:YAG laser pulses was reported.2 This approach to substituting a laser system for ultrasound energy phacoemulsification has evolved over the years. Currently, the nanosecond laser system uses an indirect delivery method (Figure 1)—that is, laser energy is not applied directly to the cataract. Instead, the laser beam strikes a titanium plate located at the end of the tip of the handpiece in the vicinity of an opening. The energy released then fragments the cataract indirectly, via a process called photofragmentation.
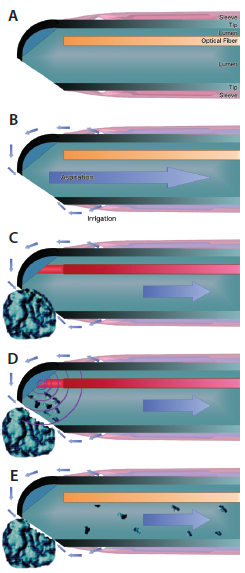
Figure 1. The titanium tip of the Nano-Laser handpiece contains a laser optical fiber (A). Irrigation liquid travels to the tip in the silicone sleeve and is aspirated via the opening that is in an off-center position (B). The nanosecond laser pulse propagates within the optical fiber and hits an angled titanium plate located at the front of the tip (C). Plasma formation forms a shockwave that causes photofragmentation of the lens material (D). The fragments are then aspirated through the titanium tip (E).
I found that the laser systems used in this approach until recently faced a significant limit, in that they were unable to effectively fragment cataracts with LOCS of grades IV or V. This was seen only as a theoretical limit, given that, based on the principles of physics, the harder the cataract, the more efficient this technique should be.
Several recent improvements now allow harder cataract formations—up to LOCS IV—to be efficiently removed. These improvements include the design of the handpiece (Figure 2) and the laser of the »Cetus Nano-Laser system (A.R.C. Laser), the use of a »Centurion Vision System with Active Fluidics Technology (Alcon) for irrigation and aspiration and manual laser control (Figure 3), and the refinement of the surgical techniques used.
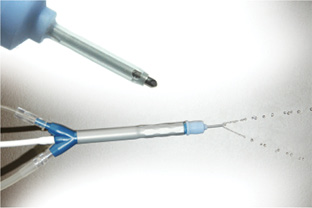
Figure 2. The disposable coaxial Nano-Laser handpiece.

Figure 3. The combination of Centurion Vision
System with Active Fluidics Technology and the Cetus Nano-Laser results in optimal fragment holding.
HARDWARE IMPROVED
The hardware improvements have followed two separate paths that have greatly improved the holding capacity of the lens fragments in front of the tip of the handpiece and, thereby, the fragmenting effect of each laser pulse.
The first line of development concerned improvements in tip design. Without increasing the overall diameter of the tip, the diameter of the optical fiber was decreased, thus increasing the aspiration capacity of the tip. This required an increase in the laser power setting from 30% to 50% of capacity, leaving a significant reserve in power should it be required during a difficult case. The second line of development concerned the configuration of the opening, which has been modified to increase the photofragmentation effect and to maximize the efficiency of the I/A system.
Other recent improvements have also decreased the Q-switch timing, yielding a faster initial rise time and therefore a faster and more efficient buildup of plasma formation. Taken together, these improvements have significantly decreased the overall release of energy within the eye required to remove a cataract.
I have tried the Nano-Laser system in combination with almost all commercially available I/A systems and prefer the Centurion.
IMPROVED SURGICAL TECHNIQUES
In tandem with the hardware improvements, I have determined that the use of two different surgical techniques can facilitate the approach to cataracts of different densities.
For cataracts with density up to LOCS II, I have found that the best technique is what I call the bowling technique (Figure 4). With the bevel pointing down, the handpiece is rotated slightly while laser pulses are applied in the nucleus, leaving the epinucleus intact, which acts as a protective shield for the posterior capsule.
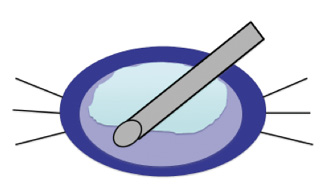
Figure 4. The bowling technique is useful in softer cataracts (LOCS I and II). The tip of the handpiece is moved in a small circle pattern with the bevel down in the nucleus, leaving the epinucleus to protect the posterior capsule. After nucleus removal, the cortex is aspirated.
In harder cataract formations of LOCS III and IV, I prefer using a drill-and-chop technique (Figure 5). With a slight drilling movement of the handpiece while the laser is fired, a central hole in the nucleus is created to at least 50% of lens thickness. Then a Neuhann chopper is used to split the nucleus in two by either chopping from the periphery toward the center of the lens or by starting inside the hole in order to crack the lens. Successful removal of these harder cataract formations without converting to ultrasound is now possible, given the hardware improvements described earlier.
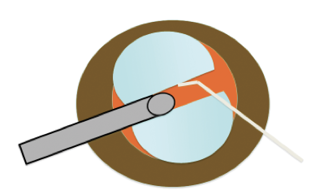
Figure 5. In harder cataracts (LOCS III and IV) a drill-and-chop technique is preferred. A slight rotating movement of the laser handpiece allows the laser to create a hole in the center of the nucleus. This is used as a basis to chop the lens with a Neuhann chopper. After chopping, the particles are fragmented with the tip of the bevel positioned parallel to the iris plane.
CLINICAL RESULTS
The Cetus Nano-Laser causes minimal to no dispersion of thermal or mechanical energy in the anterior chamber during surgery. The total amount of energy released is on the order of 8 to 10 times lower than that used in ultrasound phacoemulsification. The limited action of the nanosecond laser wave also has the advantage of not producing areas where the energy introduced within the eye is summed, thus decreasing the risk of damaging surrounding tissue (Figure 6).
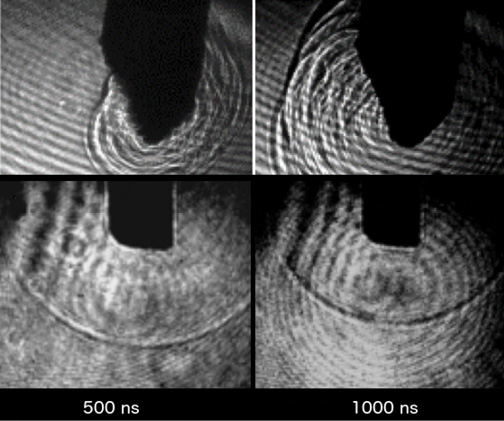
Figure 6. Images acquired with a high-speed video camera. The nanosecond laser shockwave is strongest immediately next to the handpiece tip (top row) and, unlike the ultrasound shockwave (bottom row), it does not propagate three-dimensionally. The ultrasound shockwave has a spherical front that propagates from the tip of the phaco needle in all directions and for a greater distance than the nanosecond laser wave.
The Nano-Laser handpiece offers several advantages. The single-use handpiece weighs only 30 g and has a more ergonomic design than ultrasound handpieces. The tip has no moving parts and no heat transfer to the sleeve, thus reducing damage to corneal tunnels. The system fires at a frequency on the order of 5 to 10 Hz, as compared with the 42,000 Hz of an ultrasound system. Considering the limits in response time of even the most experienced surgeons, this should result in reducing the emission of unnecessary energy when the tip is not engaged within the eye and when engaging surrounding tissue.
The system also produces a less turbulent flow and lacks the summation effects of ultrasound systems, which should lead to decreased collateral tissue damage. Overall, these advantages reduce the amount of tissue damage and maximize endothelial cell protection, which should result in a reduced risk of infection and more rapid visual rehabilitation.3
The principal advantages of a nanosecond laser system have been reported in the literature,4-6 including significantly lower levels of endothelial cell loss at 1 year postoperative and faster recovery of corneal thickness to preoperative levels in patients who underwent the Nano-Laser procedure in comparison with phacoemulsification patients.4
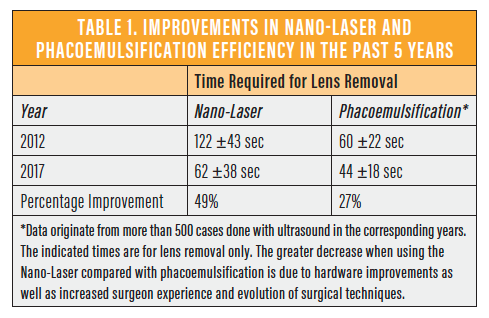
Previous improvements in the overall time required for lens removal with both nanosecond laser and ultrasound systems indicate that, while the evolution of ultrasound systems is slowing, the newer Nano-Laser system presents latitude for improvement (Table 1).
CONCLUSION
My experience performing close to 4,000 cataract surgeries with the Cetus Nano-Laser system, including numerous hard cataracts, has been positive. I have found that, with the Cetus system, the entire cataract surgery can be performed using only disposable instruments and with a significantly lower amount of energy introduced within the eye compared with phacoemulsification and with previous versions of the laser. I consider cataract surgery with the Nano-Laser to be my preferred technique when maximal protection of the corneal endothelium is needed, for example in patients with previous penetrating or lamellar keratoplasty or those with advanced cornea guttata or Fuchs endothelial dystrophy.
1. Nagy Z. Application of an integrated OCT in novel femtosecond laser cataract surgery. Paper presented at: The 14th Winter ESCRS Meeting; February 14, 2010; Budapest, Hungary.
2. Dodick JM, Sperber LTD, Lally JM, Kazlas M. NeodymiumYAG laser phacolysis of the human cataractous lens [case report]. Arch Ophthalmol. 1993;111:903-904.
3. Kanellopoulos AJ. All-laser bladeless cataract surgery, combining femtosecond and nanosecond lasers: A novel surgical technique. Clinical Ophthalmology. 2013;7:1791-1795.
4. Tanev I, Tanev V, Kanellopoulos AJ. Nanosecond laser–assisted cataract surgery: Endothelial cell study. J Cataract Refract Surg. 2016;42(5)725-730.
5. Mastropasqua L, Mattei PA, Toto L, Mastropasqua A, Vecchiarino L, Falconio G, Doronzo E. All laser cataract surgery compared to femtosecond laser phacoemulsification surgery: Corneal trauma. Int Ophthalmol. 2017;37(3):475-482.
6. Sauder G, Ruf E, Moedl S, Thyzel R. Nanosecond laser cataract surgery in LOCS III grade 4 and 5: A case series. Asia Pac J Ophthalmol. 2017;6(5):425-428.




