CASE PRESENTATION
An 85-year-old man presented for a cataract surgery evaluation. The patient reported a bilateral reduction in visual acuity that was worse in the right eye. An examination found bilateral cataracts. BCVA was 20/50 OD with a manifest refraction of -0.25 -0.5 x 117º and 20/32 OS with a manifest refraction of -0.50 D.
Although retired, the patient had an active lifestyle that included regular games of golf, driving during the day and at night, and taking cruises. He used a computer and mobile phone and enjoyed reading magazines and newspapers. He expressed a desire to be free of glasses after surgery.
Cataract surgery with the implantation of a Lentis MPlus X IOL (Oculentis) was performed on each eye. I recommended this lens because I find that patients typically experience little in the way of postoperative dysphotopsias with it and achieve good reading vision with its 3.00 D add. This IOL is made of a hydrophilic acrylic and has a hydrophobic surface.
Three months after surgery, uncorrected distance visual acuity (UDVA) was 20/25 OU, BCVA was 20/25 OD and 20/20 OS, and uncorrected near visual acuity was 20/32 OD and 20/25 OS. Two years after surgery, the patient underwent an Nd:YAG laser capsulotomy in the left eye by another surgeon.
One year after the capsulotomy, the patient returned to the clinic and reported decreased visual acuity in the left eye. Upon examination, UDVA was 20/20 OD and 20/50 OS. The IOL in the right eye was clear, but the IOL in the left eye was cloudy from calcification of its surface (Figure).
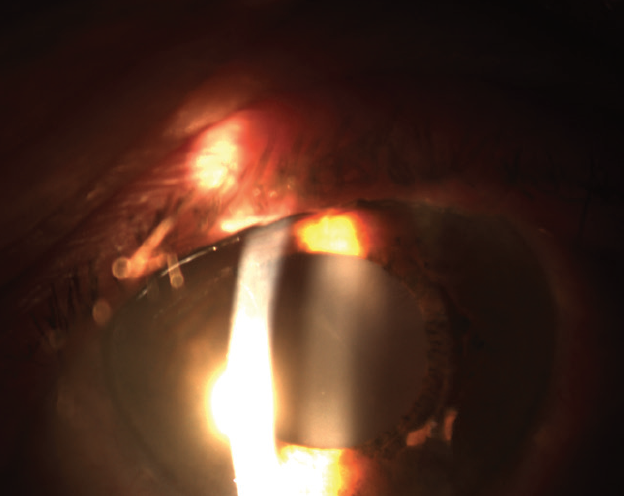
Figure. Calcification of the IOL in the left eye was evident.
How would you proceed? If you would perform an IOL exchange, what style of lens would you choose as a replacement and why? Are there any special concerns you have about this case?
—Case prepared by Magda Rau, MD

GERD U. AUFFARTH, MD, PHD, FEBO
Cases similar to this one are not unusual in Germany. Calcification of hydrophilic acrylic IOLs, this lens model in particular, have been reported frequently. The German regulatory body has issued several safety advisories on this matter. In a recent publication by The David J. Apple International Laboratory of Ocular Pathology, my colleagues and I reported that IOL calcification is by far the most frequent reason for IOL explantation.1 There is a difference between primary calcification caused by the manufacturing process and secondary calcification caused by additional external parameters such as air/gas exposure to the hydrophilic IOL during Descemet membrane endothelial keratoplasty or pars plana vitrectomy with air/gas tamponade.2,3
After bilateral implantation, it is not unusual for only one multifocal IOL to become opacified, but this patient’s situation poses three main challenges.
- No. 1: How to replace the explanted IOL and still provide multifocality or depth of focus comparable to that on the unaffected side.
- No. 2: How to explant an IOL that has been in the capsular bag for several years. The design of the Oculentis MF series includes closed-loop haptics in which lens epithelial cells can grow, thereby strongly fixating the lens in the capsular bag.
- No. 3: The open posterior capsule. Eyes that receive a hydrophilic IOL often develop posterior capsular opacification (PCO) earlier than eyes that receive IOLs manufactured out of other materials, but surgeons also frequently mistake IOL opacification for PCO.
I am very familiar with IOL explantation, especially of hydrophilic IOLs because these cases are frequently referred to me. In general, it is fairly easy even after several years to explant hydrophilic IOLs when the capsular bag is intact. The soft material is forgiving, the capsular bag can be cleaned, and in-the-bag implantation of another IOL is possible. When a bifocal IOL with a 3.00 D near addition is being explanted, it is possible to achieve multifocality with a hydrophobic acrylic IOL using the same optic design such as an Acunex VarioMax IOL (Teleon Surgical) or a small-aperture IOL such as an IC-8 (AcuFocus)—the latter is an approach that is used as a refractive procedure to provide spectacle independence.4
In this case, however, a posterior capsulotomy was performed for PCO 1 year ago. As a result, there is a high risk that the posterior capsule will be torn apart and vitreous will come forward during IOL explantation. It is sometimes possible to start with a pars plana approach and, by performing anterior and core vitrectomies, to minimize the danger of vitreous prolapse during surgery and to preserve the capsular bag for in-the-bag IOL implantation. These circumstances, however, are rare.
A safer approach is to explant the IOL out of the bag carefully without further damaging the bag diaphragm using a cohesive OVD. Slow, meticulous loosening of the haptics is important because there are strong adhesions and fibrosis might have formed around the closed-loop haptics. Sometimes, the haptics must be amputated and remain in the periphery of the capsular bag to minimize damage.
Unfortunately, no multifocal IOL for sulcus fixation is available or approved in most regions of the world. A three-piece monofocal IOL usually must be implanted in the sulcus with optic capture in the (intact) capsulorhexis. Theoretically, a transscleral fixation or even iris suturing of a multifocal IOL may be possible in selected cases, but the risk-benefit ratio would definitely tilt toward risk.

GILLES LESIEUR, MD
This is a difficult case, and more information on the cornea and retina would be helpful. If this patient’s left eye is dominant, after explantation of the Lentis MPlus X IOL through a small incision, I would consider replacing the current IOL with the same lens because the manufacturer has resolved the calcification problem.
Alternatively, an extended depth of focus IOL such as an IsoPure (Beaver-Visitec International/PhysIOL) could be implanted to provide crisp distance and intermediate visual acuity (1.00 D defocus). A vitrectomy may be warranted as well as an optic in the bag and haptics in the sulcus, depending on the importance of the posterior capsulotomy and possible instability of the IOL in the bag.
If the patient’s left eye is nondominant, I would implant an extended depth of focus IOL such as a Synthesis Plus (Cutting Edge) or a Lucidis (Swiss Advanced Vision) to provide greater defocus (1.50 to 2.00 D) with a small reduction in distance visual acuity and contrast sensitivity. I would recommend also having an Artisan Aphakia iris-fixated IOL (Ophtec), with power calculated for placement in a posterior position, in the OR in case of capsular bag injury.

RICHARD PACKARD, MD, DO, FRCS, FRCOPHTH
The problem of lens opacification due to surface calcification is well recognized with hydrophilic IOLs, and the cause is not generally identifiable. The calcified IOL in this case, however, was manufactured by the German company Oculentis, and calcification was reported with many hundreds of this company’s lenses across Europe from a particular series of batches. I was involved in helping to elucidate the cause of this calcification, which was found to be phosphate in the detergent used to treat the IOLs.
The visual acuity in this patient’s left eye is significantly impaired, and it will get worse over time as the mineralization continues. An IOL exchange is therefore required. What will make surgery more hazardous in this case is the presence of an open posterior capsule. Moreover, the haptics of these plate haptic lenses are quite stiff, which will make mobilization from the capsular bag difficult.
Before lens removal, a full and frank conversation is required to inform the patient of the potential risks of surgery and to set realistic expectations. In all likelihood, it will not be possible to replace the current lens with another multifocal IOL. Even if it is possible to implant a multifocal IOL, a different design will be required. If the right eye is dominant, then a small amount of myopia (on the order of -1.00 D) may provide the least disturbing visual outcome.
During surgery, copious amounts of a dispersive OVD should be used to open the bag and tamponade the anterior hyaloid membrane. Approaching the lens at 90º to its long axis should allow the edge of the IOL to be lifted and each of the plate haptics to be flexed out of the capsular bag in turn. The use of Packer/Chang IOL cutters (MicroSurgical Technology) and lens forceps can make it easier to cut an IOL along the long axis. The pieces can then be removed.
If the capsular bag opening is small and the zonules are intact, it may be possible to place another multifocal lens in the bag. If not, however, and if the patient has agreed to receive a monofocal lens, then a three-piece IOL may be placed in the sulcus and the optic captured.

WHAT I DID: MAGDA RAU, MD
Although many factors are thought to be involved in IOL calcification, it occurs mainly with hydrophilic lenses. In an investigation launched by Oculentis, a materials chemist simulated conditions in the anterior chamber in an effort to identify factors in IOL calcification. The study included several IOLs from different manufacturers as well as IOLs manufactured by Oculentis. The chemist found that particular batches of Oculentis lenses were more prone to calcification than other batches of the same lenses. As Dr. Packard notes, the issue was eventually traced back to the detergent, which contained phosphate, that was used to clean the lenses. The company recalled all of the lenses from the affected batches, but many hundreds of eyes across the world had already received these lenses and ultimately required IOL exchanges.
The patient in this case required explantation of the calcified IOL. Unfortunately, the ophthalmologist who performed the capsulotomy created a large opening in the capsule, which had the potential to make explantation difficult. The capsulorhexis, however, was about 4.8 mm in diameter, and I thought the remaining capsule would provide sufficient support for a three-piece multifocal IOL placed in the sulcus. Nevertheless, I devised a backup plan. If the capsulorhexis, part of the anterior capsule, or the whole capsular bag were to be damaged during explantation of the IOL, transscleral fixation of an IOL would be performed. To do so, I would create Hoffman pockets, and both needles of a double-armed 9-0 polypropylene suture would be used to suture the IOL to the sclera. Because it can be difficult to achieve perfect centration of a multifocal IOL with this technique, a monofocal lens would be implanted instead. Although this would not fulfill the patient’s request for spectacle independence in both eyes, based on my experience, I anticipated that the two eyes working together would provide spectacle independence. The informed consent process included a discussion of the possible need for a monofocal IOL and for additional surgery in the future.
Watch It Now
A 2.8-mm perilimbal incision was created in an effort to prevent lens fragments from causing uncontrolled enlargement of the clear corneal incision, which could induce astigmatism. An OVD was injected into the anterior chamber to protect the endothelium during IOL removal. More of the OVD was injected with a hydrodissection cannula into the capsular bag and under the lens. During this maneuver, the plate haptics of the IOL were slowly manipulated out of the capsular bag, into the anterior chamber, and in front of the iris. The long axis of the IOL was rotated so that it was in line with the incision. The IOL was stabilized with a spatula and cut into two pieces with Wecker scissors. By cutting the IOL through its long axis, I was able to avoid enlarging the incision. This explantation technique works well in my hands.
My plan was to remove each piece of the IOL separately without touching the capsulotomy or the capsular bag. Unfortunately, the lens had not been completely bisected, and a thin bridge of material remained. As I attempted to remove the first lens piece, the entire IOL rotated. I was able to remove the lens in a single step, but one of its edges touched the capsulotomy. An injection of dilute triamcinolone acetonide into the anterior chamber revealed some vitreous, so a bimanual anterior vitrectomy was performed.
A three-piece multifocal IOL was implanted in the ciliary sulcus, and good centration was achieved. UDVA was 20/32, which corrected to 20/25 with a manifest refraction of -0.50 D. The patient was satisfied with the outcome.
1. Neuhann T, Yildirim TM, Son HS, Merz PR, Khoramnia R, Auffarth GU. Reasons for explantation, demographics, and material analysis of 200 intraocular lens explants. J Cataract Refract Surg. 2020;46(1):20-26. Erratum in: J Cataract Refract Surg. 2020;46(7):1068.
2. Yildirim TM, Auffarth GU, Łabuz G, Bopp S, Son HS, Khoramnia R. Material analysis and optical quality assessment of opacified hydrophilic acrylic intraocular lenses after pars plana vitrectomy. Am J Ophthalmol. 2018;193:10-19.
3. Giers BC, Tandogan T, Auffarth GU, et al. Hydrophilic intraocular lens opacification after posterior lamellar keratoplasty – a material analysis with special reference to optical quality assessment. BMC Ophthalmol. 2017;17(1):150.
4. Son HS, Khoramnia R, Yildirim TM, Baur I, Labuz G, Auffarth GU. Functional outcomes and reading performance after combined implantation of a small-aperture lens and a segmental refractive bifocal lens. J Refract Surg. 2019;35(9):551-558.




