Simplified Regimens, Extended Delivery Options Among Recent Advances in Steroid Delivery for Cataract Surgery
By Damien F. Goldberg, MD

Steroid medications are an integral part of how we care for patients after cataract surgery. I still recall the drop packs my attendings used to give our cataract surgical patients when I started residency. Every patient left with a postoperative pack containing an eye shield, sunglasses, an antibiotic drop, a steroid drop, and an NSAID drop.
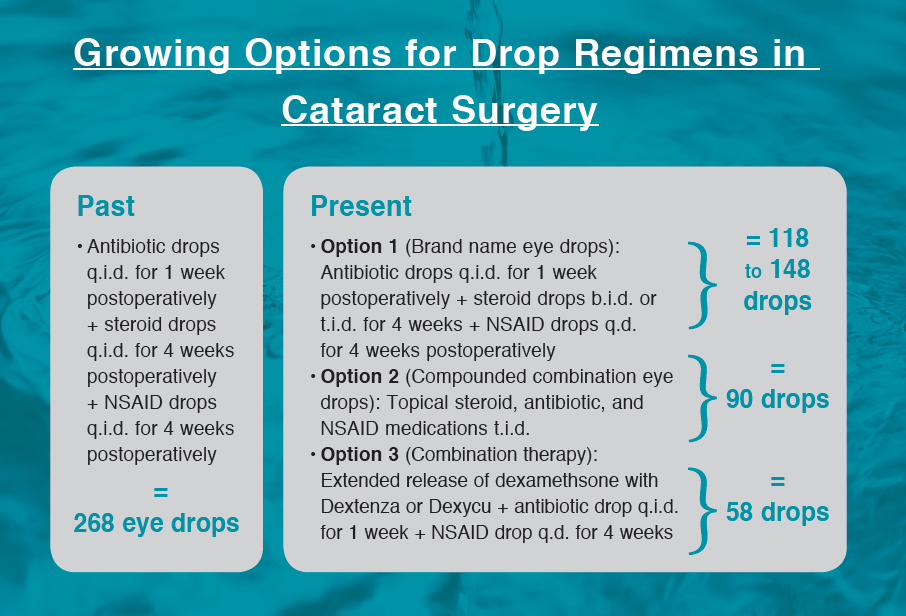
Prednisolone acetate ophthalmic suspension 1% (Pred Forte, Allergan) was a popular choice for the steroid, and ketorolac tromethamine ophthalmic solution 0.5% (Acular, Allergan) was popular for the NSAID. Strong empirical data supported the use of both steroid and NSAID as a significant way to reduce the potential side effect of cystoid macular edema after cataract surgery.1 However, prescribing triple eye drop therapy had its issues. Not every patient was willing or would remember to use all three medications q.i.d. Not to mention, 268 drops per eye (assuming the antibiotic was taken for 1 week and the steroid and NSAID each for 4 weeks) from three bottles with three different preservatives was rough on the corneal surface and complicated the healing process.
ADVANCES IN DOSING, DELIVERY
Since that time, through advances in drug delivery and posology, the dosing schedules for both antibiotic and antiinflammatory drops have been significantly simplified. Difluprednate ophthalmic emulsion 0.05% (Durezol, Alcon) was the first steroid that allowed us to reduce dosing. It was shown to have double the potency of prednisolone acetate, and it remains the most potent topical steroid in our arsenal. It can be dosed t.i.d. rather than q.i.d.2
More recently, two formulations of loteprednol etabonate, a softer steroid, have become available, and each helps to improve bioavailability in different ways. A gel formulation, loteprednol etabonate ophthalmic gel 0.38% (Lotemax SM, Bausch + Lomb), increases residence time for the drug.3 And a formulation using nanoparticles, or mucus-penetrating particle technology, helps the drug penetrate to target tissues. Both of these loteprednol formulations can be dosed b.i.d.
Another familiar formulation, fluorometholone acetate ophthalmic suspension 0.1% (Flarex, now Eyevance Pharmaceuticals), has had a resurgence and gained popularity.
SIMPLIFY: Multiple medications, one drop
These medications decrease the frequency of steroid treatment, but navigating three different postoperative medications remains a compliance and cost barrier for patients. Many physicians who may have previously preferred to prescribe brand name drugs have noted the burden of prices in the current complex health care landscape. In the search for alternative solutions, many practices have begun routine use of compounded combination topical steroid, antibiotic, and NSAID medications. One option is prednisolone phosphate 1%/gatifloxacin 0.5%/bromfenac 0.075% (Omni, OSRX Pharmaceuticals), prescribed t.i.d. for 1 month.
Advantages of the compounded approach include improving patient compliance, preventing health plans or pharmacies from changing or denying prescribed medications, and eliminating staff time spent dealing with callbacks from health insurance plans. Additionally, compounded drops can considerably lower patients’ out-of-pocket costs. Moreover, one study found that inflammation was reduced in patients treated with a compounded combination. In a randomized, double-masked prospective US multicenter study,4 an earlier version of the compounded prednisolone/gatifloxacin/bromfenac demonstrated a 50% reduction in summed ocular inflammation score, compared with a 23% reduction in control patients using separate brand name medications: Pred Forte, moxifloxacin ophthalmic solution (Vigamox, Novartis), and nepafenac ophthalmic suspension 0.3% (Ilevro, Novartis). The success of the compounded drops has been attributed to the innovative simplicity of freeing patients to administer fewer drops postoperatively.
Some cataract surgeons have also begun to use compounded intracameral injections of a corticosteroid and fluoroquinolone antibiotic combination, dexamethasone phosphate 0.1%/moxifloxacin HCl 0.5% sterile ophthalmic injection (Omni Intracameral Injection, OSRX Pharmaceuticals).
SUSTAINED RELEASE DELIVERY IS HERE
In the past year, we have also seen the introduction of two extended release options for steroid drug delivery that look promising to treat postoperative inflammation. The dexamethasone ophthalmic insert 0.4 mg (Dextenza, Ocular Therapeutix) is an intracanalicular corticosteroid insert placed in the punctum.5 The dexamethasone intraocular suspension 9% (Dexycu, EyePoint Pharmaceuticals) is an intracameral option.6
There is a great deal of enthusiasm to see the development of new steroid drug delivery options such as these, but working through the details of the cost barrier with these new drug delivery options can be challenging. (Editor’s note: For more on this topic, see “The Economics of Transitional Pass-Through Status.”) Once drug coverage for these agents is widely established, it will no doubt lead to greater adoption.
CONCLUSION
The traditional regimen of prescribing steroid eye drops remains the main method of postoperative cataract surgery care, but new technologies and compounded formulations such as those described here offer great promise.
When used appropriately, there is potential for improving compliance, comfort, and reliability. Innovation in pharmacotherapy is continual, and these new options have allowed us to greatly improve the method of delivering steroid medications for our patients’ care.
1. Wittpenn J, Silverstein S, Hunkler J, et al. A masked comparison of Acular LS plus steriod versus steroid alone for the prevention of macular leakage in cataract patients. Am J Ophthalmol. 2008;146(4):554-560.
2. Smith S, Lorenz D, Peace J, McLeod K, Crockett RS, Vogel R. Difluprednate ophthalmic emulsion 0.05% (Durezol) administered two times daily for managing ocular inflammation and pain following cataract surgery. Clin Ophthalmol. 2010;4:983-991.
3. Phillips E, Coffey MJ, Shawer M. Viscoelastic and dissolution characterization of submicron loteprednol etabonate ophthalmic gel, 0.38%. Invest Ophthalmol Vis Sci. 2015;56(7):1525.
4. Fram N, Masket S, Shamie N, et al. Prospective US multicenter study of safety and efficacy of combination drop prednisolone phosphate, moxifloxacin, and ketorolac in treating post-op inflammation and pain after cataract surgery. Paper presented at: the ASCRS Annual Meeting; May 5-9, 2017; Los Angeles.
5. Tyson SL, Bafna S, Gira JP, et al; Dextenza Study Group. Multicenter randomized phase 3 study of a sustained-release intracanalicular dexamethasone insert for treatment of ocular inflammation and pain after cataract surgery [published correction appears in J Cataract Refract Surg. 2019;45(6):895]. J Cataract Refract Surg. 2019;45(2):204-212.
6. Donnenfeld ED, Solomon KD, Matossian C. Safety of IBI-10090 for inflammation associated with cataract surgery: Phase 3 multicenter study. J Cataract Refract Surg. 2018;44(10):1236-1246.
A Trend Toward Reduced Steroid Use in Refractive Surgery
By Beeran Meghpara, MD

Most refractive surgeons would agree that topical steroids play a role in patient care after corneal refractive surgery. But this is where the consensus ends; there is significant variation in the specific regimens discussed in the literature and mentioned anecdotally by colleagues. The type of steroid used, the frequency of application, and the duration of treatment vary widely. There are also different considerations depending on whether we are performing PRK or LASIK. This article addresses some of those considerations.
CONSIDERATIONS IN PRK
In the pivotal studies that led to the FDA approval of PRK in 1995, the steroid fluorometholone 0.1% was tapered postoperatively over the course of about 4 months. The nature of the PRK procedure leads to injury of the corneal epithelium and basement membrane, inciting the release of inflammatory mediators and cytokines. An extended postoperative course of topical corticosteroids reduces this inflammatory response and thereby may decrease corneal haze formation and myopic regression.1-3
A 4-month postoperative medication regimen, however, can be burdensome to patients. Fortunately, evolution in PRK technique and laser technology have reduced the risk of postoperative haze formation and, in turn, influenced the duration of postoperative steroid use. Today, it is less imperative to have a prolonged course of steroids after PRK. The use of mitomycin C at the time of surgery4 and the larger ablation diameter and small beam flying spot of modern lasers5 both help to decrease the risk of haze formation. This combination has increased my comfort in using a shorter steroid course.
My preferred postoperative regimen is to use a steroid ester such as flurometholone acetate 0.1% or loteprednol etabonate 1% (Lotemax, Bausch + Lomb) tapered over the course of 8 weeks. I have found that this protocol lowers the risk of postoperative IOP rise.
Another option is the use of no-drop steroid delivery to the ocular surface with a sustained delivery device such as the dexamethasone ophthalmic insert 0.4 mg. This intracanalicular insert delivers a controlled dose of preservative-free dexamethasone to the ocular surface over the course of 30 days. Anecdotal reports have shown success with its use following PRK. A prospective, open-label, randomized, contralateral-eye-controlled study is under way to determine patient preference and the incidence and grade of postoperative corneal haze with the dexamethasone ophthalmic insert versus prednisolone acetate 1% q.i.d. for 1 week followed by b.i.d. for 1 week after bilateral PRK.6
CONSIDERATIONS IN LASIK
LASIK incites a significantly lower inflammatory response compared to PRK, and therefore most refractive surgeons are comfortable prescribing a lower dose of steroids postoperatively. One randomized trial showed that topical steroids did not play a beneficial role in postoperative recovery following uncomplicated LASIK.7
A reasonable alternative, and a consensus among practitioners, is to prescribe steroids in the first week after LASIK. The benefit of extending treatment beyond that time is debatable, and prolonged steroid treatment can lead to the potential complication of pressure-induced stromal keratopathy (PISK).8,9 Often resembling and frequently misdiagnosed as diffuse lamellar keratitis (DLK), PISK is caused by fluid accumulation in the flap interface secondary to steroid-related increase in IOP. When treated as DLK with more topical steroids, the complication can worsen. Left untreated, however, PISK can lead to potential glaucomatous damage of the optic nerve and loss of visual field.
My current steroid regimen with LASIK is flurometholone acetate 0.1% every 2 hours the day of surgery and q.i.d. for 1 week after surgery. Another option is loteprednol etabonate 1% gel. The gel vehicle results in homogeneous steroid delivery without the need to shake the bottle. However, some reports have shown that newer-generation steroids with more viscous vehicles can be associated with a higher rate of DLK. Care should therefore be taken not to allow these drop formulations to come into contact with the cornea preoperatively or the exposed stromal bed intraoperatively, but rather used after the LASIK flap is repositioned. When used after the LASIK flap is back in place, steroid gels or emulsions do not appear to increase the risk of DLK.10
I also prescribe a topical antibiotic for 1 week after LASIK. Another option is to use a compounded medication containing both antibiotic and topical steroid. This method of postoperative patient care has been gaining popularity in the cataract surgery space; however, there is some concern for prolonged use of a topical antibiotic over the typical 3- to 4-week steroid-tapering course after cataract surgery. With LASIK, on the other hand, because of its shorter steroid course (generally about 1 week), use of a commercially available compounded mixture of prednisolone acetate 1% and moxifloxacin (Pred-Moxi, ImprimisRx) can be effective, safe, and convenient for patients.
CONCLUSION
Overall, in recent years, we have witnessed a trend toward using less steroids after refractive surgery. As technology and technique have evolved, the risk of inflammation-related complications has decreased. This has not yet eliminated the need for postoperative steroids, but new delivery methods for topical steroids such as sustained release inserts and combined compounded medications can improve patient convenience and compliance with medication instructions.
1. Margo JA, Munir WM. Corneal haze following refractive surgery: a review of pathophysiology, incidence, prevention, and treatment. Int Ophthalmol Clin. 2016;56:111-125.
2. Marques EF, Leite EB, Cunha-Vaz JG. Corticosteroids for reversal of myopic regression after photorefractive keratectomy. J Refract Surg. 1995;11:S302-S308.
3. Pakbin M, Khabazkhoob M, Pakravan M, et al. Duration of topical steroid application after photorefractive keratectomy with mitomycin C. J Cataract Refract Surg. 2020;46(4):622-632.
4. Majmudar PA, Schallhorn SC, Cason JB, et al. Mitomycin-C in corneal surface excimer laser ablation techniques: a report by the American Academy of Ophthalmology. Ophthalmology. 2015;122(6):1085-1095.
5. Rajan MS, O’Brart D, Jaycock P, Marshall J. Effects of ablation diameter on long-term refractive stability and corneal transparency after photorefractive keratectomy. Ophthalmology. 2006;113:1798-1806.
6. The RESTORE Study, a randomized, controlled, masked (reading center) prospective study. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04396990. Accessed June 23, 2020.
7. Price FW Jr, Willes L, Price M, Lyng A, Ries J. A prospective, randomized comparison of the use versus non-use of topical corticosteroids after laser in situ keratomileusis. Ophthalmology. 2001;108(7):1236-1245.
8. Belin MW, Hannush SB, Yau CW, Schultze RL. Elevated intraocular pressure-induced interlamellar stromal keratitis. Ophthalmology. 2002;109(10):1929-1933.
9. Davidson RS, Brandt JD, Mannis MJ. Intraocular pressure-induced interlamellar keratitis after LASIK surgery. J Glaucoma. 2003;12(1):23-26.
10. ASCRS issues medication alert for LASIK and PRK. Eyewire. March 2013. https://eyewire.news/articles/20130215-ascrs_issues_medication_alert_for_lasik_and_prk/. Accessed July 1, 2020.
Sustained Release Glaucoma Drugs May Offer Benefits in Compliance and Continuous Dosing
By Manjool Shah, MD

In 1985, a prospective randomized trial formally demonstrated improved outcomes from conventional filtering surgery when topical steroids were applied,1 and since that time corticosteroid therapy has become a mainstay and the standard of care in glaucoma surgery. But recent changes in the glaucoma surgical landscape and innovations in drug delivery have also begun to change our approaches to postoperative antiinflammatory therapy.
Concerns regarding the promotion of fibroblast proliferation by steroids have also been raised. A retrospective clinical study found that subconjunctival dexamethasone, injected over the area of a filtering bleb, increased encapsulation rates compared with an injection 180° away from the bleb.2 Those investigators suggested that this may be a result of a biphasic response to steroids by episcleral fibroblasts, in which a low dose of steroids support fibroblast growth but a high dose can inhibit fibroblast proliferation.
Corticosteroid therapy in glaucoma care is further complicated by the specter of steroid-induced ocular hypertension. This phenomenon, certainly a cause of elevated IOP in glaucoma patients, is also observed in normal eyes to a lesser degree and at a lower frequency.3 Although steroid response is typically self-limited with discontinuation of therapy, the elevation of IOP can be dangerous in some cases.
SUSTAINED RELEASE
The challenges of fine-tuning corticosteroid therapy after glaucoma surgery are further compounded by patient compliance and adherence. Fortunately, innovations in the field of sustained release pharmacotherapy might mitigate some of these concerns.
The theoretical benefits of sustained release therapy go beyond the issue of adherence, in fact. Steady and consistent delivery of steroids around the clock may provide better results than pulsatile and inconsistent topical therapy, delivered only when the patient is awake and remembers to take the drops.
In a small case series, subconjunctival placement of the sustained release dexamethasone intravitreal implant 0.7 mg (Ozurdex, Allergan) after trabeculectomy demonstrated a favorable wound healing profile,4 suggesting a possible benefit of additional investigation in this space.
Two newer players in sustained release corticosteroid delivery may therefore represent promise in the postoperative management of both conventional glaucoma surgery and MIGS. Clinical experience and clinical trial data have demonstrated that adjunctive MIGS procedures do not promote significant additional inflammation when combined with cataract surgery as compared to cataract surgery alone.5
Therefore, it may be that these new intracameral and intracanalicular sustained release steroid formulations, dexamethasone intraocular suspension 9% and the dexamethasone ophthalmic insert 0.4 mg, may offer benefit for postoperative antiinflammatory therapy for internal, Schlemm canal-based MIGS surgeries.
In the phase 3 clinical trial evaluating the safety and efficacy of the dexamethasone intraocular suspension,6 although an 11% incidence of elevated IOP was reported, only one patient required IOP-lowering therapy for a transient duration. No patient in the phase 3 clinical trial evaluating the dexamethasone ophthalmic insert demonstrated an elevation in IOP that was concerning as a true steroid response.7 Therefore, both technologies seem to offer promise for use in combination with routine MIGS procedures.
STILL INVESTIGATIONAL
The application of these newer technologies in conventional glaucoma surgeries and in subconjunctival MIGS procedures such as the Xen Gel Stent (Allergan) remains investigational and the results anecdotal. Products such the sustained release dexamethasone innovations discussed here have been formulated for a drug delivery timeline most appropriate for cataract surgery, typically with the controlled release of therapy over approximately 1 month. Subconjunctival glaucoma surgery, whether conventional or MIGS-based, often requires steroid therapy over 2 to 3 months, so the available technologies on their own may not be sufficient. However, there may be independent value in their steady and continuous drug delivery. This has yet to be investigated in the context of glaucoma surgery.
Although old concepts regarding corticosteroids in glaucoma surgery still apply, new technologies are offering promise, potentially improving patient adherence and comfort and reliability. The hope is that these innovations in pharmacotherapy will provide improvement in glaucoma surgical outcomes as well.
1. Starita RJ, Fellman RL, Spaeth GL, Poryzees EM, Greenidge KC, Traverso CE. Short- and long-term effects of postoperative corticosteroids on trabeculectomy. Ophthalmology. 1985;92(7):938-946.
2. Loftfield K, Ball SF. Filtering bleb encapsulation increased by steroid injection. Ophthalmic Surg. 1990;21(4):282-287.
3. Becker B, Mills DW. Corticosteroids and intraocular pressure. Arch Ophthalmol. 1963;70:500-507.
4. Furino C, Boscia F, Cicinelli MV, Sborgia A, Alessio G. Subconjunctival sustained-release dexamethasone implant as an adjunct to trabeculectomy for primary open angle glaucoma. Indian J Ophthalmol. 2016;64(3):251-252.
5. Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118:459-467.
6. Donnenfeld ED, Solomon KD, Matossian C. Safety of IBI-10090 for inflammation associated with cataract surgery: Phase 3 multicenter study. J Cataract Refract Surg. 2018;44(10):1236-1246.
7. Tyson SL, Bafna S, Gira JP, et al. Multicenter randomized phase 3 study of a sustained-release intracanalicular dexamethasone insert for treatment of ocular inflammation and pain after cataract surgery [published correction appears in J Cataract Refract Surg. 2019;45(6):895]. J Cataract Refract Surg. 2019;45(2):204-212.




