
When most of us think of offering refractive cataract surgery to our patients for correction of presbyopia, we are thinking of surgery with premium lenses—that is, multifocal, extended depth of focus (EDOF), or accommodating IOLs. But premium IOLs require special preparation for the patient and surgeon, and each of them has potential drawbacks.
Multifocal IOLs can, in some patients, induce dysphotpsias, reduce contrast sensitivity, and create a poor quality of vision. Additionally, they require a process of neural adaptation that, in many patients, can take months. EDOF IOLs tend to have less severe nighttime aberrations than multifocal IOLs, but they can still produce bothersome dysphotopsias. Some patients with EDOF IOLs achieve functional near vision, but many will not acquire what they consider satisfactory near vision. Accommodating IOLs are essentially monofocal IOLs, and, thus, they have the advantage of better quality night vision without significant aberrations. However, the near vision with these lenses tends to be inadequate in most patients, and there is the rare instance of Z-syndrome, which in some patients cannot be resolved without an IOL exchange.
It is because of these potential drawbacks of premium IOLs that some surgeons consider monovision as an option for refractive cataract surgery and refractive lens exchange in patients desiring correction of presbyopia.
For most patients, monovision entails providing distance vision or emmetropia in the dominant eye and near vision or myopia in the nondominant eye. Ophthalmologists have been using monovision in their patients for years, whether with contact lenses, corneal refractive surgery, or pseudophakia at the time of cataract or lens surgery. Monovision has many potential advantages over multifocal and EDOF IOLs, including limited dysphotopsias and faster neural adaptation. Further, monofocal IOLs are not as sensitive to residual astigmatism and posterior capsular opacification as premium lenses, and they can be used in eyes with borderline corneal and macular pathology.
This article outlines some of the considerations involved in offering monovision to cataract patients desiring presbyopia correction, not as an afterthought, but as a high-quality option for refractive cataract surgery.
PATIENT SELECTION AND COUNSELING
Patient selection for monovision is just as important as it is for multifocal and EDOF IOLs. Patients should have a strong desire for spectacle independence and be able to comprehend the implications and limitations of monovision. The easiest patients to recommend for monovision are those who have worn monovision previously with contact lenses. Poor candidates include patients with strong ocular dominance or an exodeviation greater than 10 prism diopters.
For patients with mild to moderate myopia in both eyes, it is essential before cataract surgery to explain the options available and to stress the loss of near vision that will occur if monofocal IOLs targeted for emmetropia are implanted in both eyes. One option for indecisive patients who decline presbyopia-correcting IOLs is to fix their dominant eye for emmetropia and then trial monovision with a contact lens for several months, with either their original myopia or reduced myopia in the nondominant eye.
A handful of studies have looked at reading ability and success rates with pseudophakic monovision. One concern following monovision is the possibility of impaired reading ability. However, a study by Ito and Shimizu demonstrated that individuals with pseudophakic monovision actually had better critical size and reading results than individuals implanted with refractive multifocal IOLs.1 It is important to keep in mind that this study evaluated patients with refractive multifocal IOLs, which in general provide poorer near acuity than diffractive multifocal IOLs.
HOW MUCH ANISOMETROPIA?
Greenbaum reported a high success rate with pseudophakic monovision.2 In his report, 92% of patients achieved 20/30 or better distance and J1 near unaided postoperatively, with an overall acceptance rate of 90%. Finkelman and colleagues found that the sweet spot for the nondominant eye was a target between -1.00 and -1.50 D.3 In that study, 96% of patients achieved 20/30 or better distance and 92% achieved J4 or better near. Good stereopsis and contrast sensitivity were achieved, and, in general, patients were satisfied with their results.3 Another study, by Marquess and colleagues, used a -2.00 D myopic target and found that 100% of patients achieved 20/40 or better for distance and J3 or better for near. In their study, 90% of patients also achieved J3 intermediate acuity, and overall patient satisfaction was 97%.4
The results of these various studies raise the question: “How much anisometropia is ideal?” Or, simply: “What should we be aiming for in the nondominant eye?”
Wright and colleagues found that there was a reduction in Titmus stereoacuity with monovision resulting in anisometropia of 2.00 D or more.5 Similarly, Handa et al found that binocular rivalry and strong ocular dominance resulted in asthenopia when anisometropia was 2.00 D or more with pseudophakic monovision.6 Due to these and other studies, the current recommendation for pseudophakic monovision is to aim for -1.00 to -1.50 D in the nondominant eye.
Hayashi and coworkers perhaps refined this recommendation one step further, noting that the number of patients in their study who met the criteria for successful monovision was significantly greater with a -1.50 D target than with either a -1.00 or -2.00 D target for the nondominant eye.7
In general, these recommendations should be weighed against each individual’s particular desires for postoperative functionality. A patient who desires only computer vision might do well with a -1.00 D target, whereas an individual who requires excellent close vision might need -2.00 D or more in order to achieve satisfaction.
WHICH LENS MODEL?
Another aspect of monovision that deserves attention is which IOL model to choose in order to optimize distance and near vision. Mixing and matching presbyopia-correcting IOLs is a common practice in refractive cataract surgery, and surgeons can choose from a range of multifocal, EDOF, and accommodating IOLs in the hope of maximizing each individual IOL’s benefits to cover the entire spectrum of vision for patients, from distance to near.
Similarly, for monovision, various models of monofocal IOL can be mixed and matched in order to cover the whole range of functional distances. This mixing of monofocal IOLs in particular involves the combination of spherical and aspheric IOLs.
There has been a trend for the past decade or 2 of using aspheric IOLs, with either no spherical aberration or negative spherical aberration, to yield the sharpest distance image possible. This stems from the knowledge that the cornea maintains positive spherical aberration throughout an individual’s life, while the spherical aberration in the crystalline lens changes over time. When we are young, the crystalline lens has negative spherical aberration to counter the positive spherical aberration of the cornea. In the past, placing older-style IOLs with inherent positive spherical aberration compounded the spherical aberration in the cornea, yielding greater positive spherical aberration and resulting in a greater likelihood of blurred vision and nighttime glare and halos for patients after cataract surgery. Using an IOL with negative spherical aberration, on the other hand, reduces the overall spherical aberration in the optical system and yields better quality vision.
Although this is a good approach for the distance eye in a monovision model, placing a spherical IOL in the near eye may be a better means of maximizing the patient’s range of near vision.
Rocha and colleagues studied distance, intermediate, and near acuity in eyes implanted with spherical and aspheric IOLs.8 In this study, eyes implanted with spherical IOLs had slightly better intermediate and near acuities compared with eyes implanted with aspheric IOLs (Table). This result suggested that there is an increased depth of field in eyes implanted with spherical IOLs.
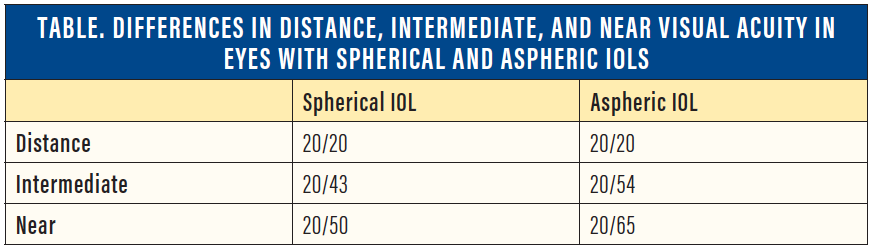
Thus, one approach for maximizing the quality of vision and depth of field in patients undergoing monovision correction would be to use an aspheric IOL in the distance eye to yield the best quality distance vision and a spherical IOL in the near eye to yield the best possible depth of field (Figure).
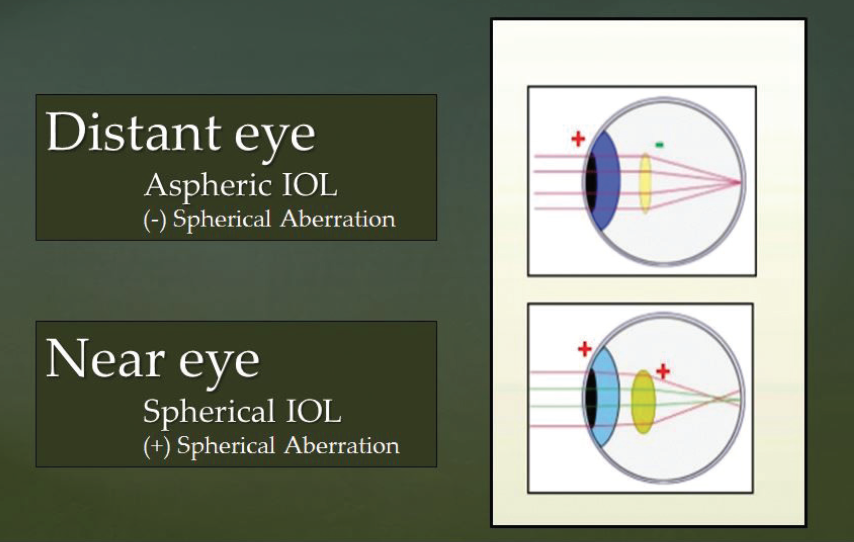
Figure. Using an aspheric IOL with negative spherical aberration in the distance eye (to yield the best quality distance vision) and a spherical IOL with positive spherical aberration in the near eye (to yield the best possible depth of field) might be an optimal strategy for monovision correction.
HIGH QUALITY, HIGH SATISFACTION
Pseudophakic monovision is a high-quality form of refractive cataract surgery. In appropriately selected patients, there is a high level of acceptance and satisfaction with monovision correction. In the distance eye, the aim should be to provide full emmetropic correction with a high-quality aspheric monofocal IOL. In the near eye, the aim should be a target between -1.00 and -1.50 D, with the consideration of placing a spherical IOL in order to maximize depth of focus.
Good candidates for monovision include highly motivated patients and especially patients who have enjoyed monovision either naturally or with the use of contact lenses. Patients with a history of strabismus or strong ocular dominance should be approached carefully, and perhaps monovision should be avoided in these patients.
Ultimately, pseudophakic monovision offers the potential for significant spectacle independence without the nighttime aberrations and loss of quality vision that can occur with multifocal and EDOF lenses. It should be an option in every ophthalmologist’s armamentarium.
1. Ito M, Shimizu K. Reading ability with pseudophakic monovision and with refractive multifocal intraocular lenses: comparative study. J Cataract Refract Surg. 2009;35:1501-1504.
2. Greenbaum S. Monovision pseudophakia. J Cataract Refract Surg. 2002;28:1439-1443.
3. Finkelman YM, Ng JQ, Barrett GD. Patient satisfaction and visual function after pseudophakic monovision. J Cataract Refract Surg. 2009;35:998-1002.
4. Marquess FF, Sato RM, Chiacchio BB, et al. Evaluation of visual performance and patient satisfaction with pseudophakic monovision technique. Arq Bras Oftalmol. 2009;72:164-168.
5. Wright KW, Guemes A, Kapadia MS, Wilson SE. Binocular function and patient satisfaction after monovision induced by myopic photorefractive keratectomy. J Cataract Refract Surg. 1999;25:177-182.
6. Handa T, Mukuno K, Uozato H, et al. Ocular dominance and patient satisfaction after monovision induced by intraocular lens implantation. J Cataract Refract Surg. 2004;30(4):769-774.
7. Hayashi K, Yoshida M, Manabe S, Hayashi H. Optimal amount of anisometropia for pseudophakic monovision. J Refract Surg. 2011;27:332-338.
8. Rocha KM, Soriano ES, Chamon W, et al. Spherical aberration and depth of focus in eyes implanted with aspheric and spherical intraocular lenses: a prospective randomized study. Ophthalmology. 2007;114:2050-2054.




