Numerous studies have demonstrated that reducing IOP slows the progression of glaucomatous optic neuropathy.1-4 Because it remains the first modifiable risk factor identified, IOP is largely the focus of glaucoma care today. Recent evidence, however, suggests that another modifiable risk factor, ocular perfusion pressure (OPP), may be involved in the multifactorial disease process of glaucoma.5-7
AT A GLANCE
- If practitioners could measure ocular blood flow noninvasively and without laborious calculations, perhaps they could treat something besides IOP. Optical coherence tomography angiography <(OCT-A)< may hold the key.
- OCT-A is a new imaging technique that detects motion contrast to create angiographic images of the optic nerve and/or retina. The technology compares differences in background OCT signal intensity to sequential interval OCT scans to map blood flow through the specific area of interest.
- The literature on OCT-A is in its infancy. Studies are needed to determine if altering ocular perfusion pressure affects glaucomatous progression.
The mathematical formula to calculate mean OPP is 2/3[diastolic BP - 1/3(systolic BP - diastolic BP)] - IOP, where BP stands for blood pressure. Unfortunately, carrying out this calculation is prone to error. At what point during the day should BP be measured? What position should the patient assume? Do antihypertensive medications cause strong diurnal changes in BP and thus OPP? These uncertainties—added to the inherent difficulty of measuring “true” IOP—limit the practicality of OPP measurements in clinical practice.
If practitioners had means to measure ocular blood flow noninvasively and without laborious calculations, perhaps they could identify modifiable risk factors besides IOP. Optical coherence tomography angiography (OCT-A) may hold the key.
THE TECHNIQUE
OCT-A is a new, emerging imaging technique that detects motion contrast to create angiographic images of the optic nerve and/or retina. The technology compares differences in background OCT signal intensity with sequential interval OCT scans to map blood flow through the specific area of interest.
Initially, Wang and colleagues demonstrated that Doppler OCT could compute blood flow, but this technique was usable mainly for large blood vessels.8 Then, Jia et al used split-spectrum amplitude-decorrelation angiography to map fine peripapillary angiography. They found that a dense, well-perfused microvascular network exists in normal optic nerve vasculature versus attenuated vasculature and a 25% decreased blood flow index in glaucomatous nerves.9 Liu and colleagues used OCT-A to look beyond nerve head perfusion and concluded that the peripapillary retina had a lower flow index and vessel density in glaucomatous versus control eyes.10
The aforementioned studies provide evidence that decreased optic nerve and peripapillary perfusion is positively correlated with visual field pattern standard deviation. More recently, Yarmohammadi and colleagues demonstrated that OCT-A vessel density has diagnostic accuracy similar to that of the commonly used retinal nerve fiber layer thickness measurement, which suggests that OCT-A has a strong potential to diagnose and stage glaucoma (Figure).11
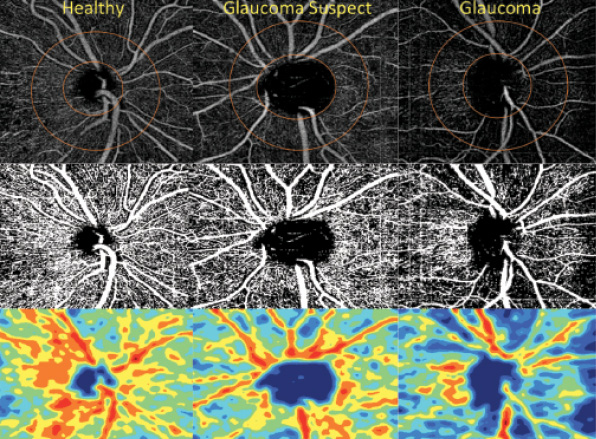
Figure. Retinal nerve fiber layer vessel density map in healthy control eyes, glaucoma suspects’ eyes, and eyes with open-angle glaucoma. Vessel density map (top), vessel density map overlaid on major vasculature (middle), vessel density color-coded (bottom). Reproduced from Yarmohammadi et al.11
Joel Schuman, MD, summarizes the past, present, and future of optical coherence tomography.
With early data showing differences in retinal vascular density (and associated retinal flow index) between glaucomatous and healthy eyes, can OCT-A provide additional tools to guide patients’ care?
FUTURE STEPS
OCT-A can give clinicians insight into a parameter besides IOP that potentially could be optimized. In patients experiencing progressive visual field loss despite adequate IOP control (including normal-tension glaucoma) and in those for whom maximal IOP-lowering measures have already been taken, perhaps the next step is understanding the pathogenesis involving optic nerve head perfusion. What measures can be investigated to improve OPP?
- Ophthalmologists can work with the patient’s primary care physician or cardiologist to ensure optimal BP control at a level high enough to maintain ocular perfusion (but obviously not so high as to cause other end organ damage).
- With physicians’ BP goals for patients with systemic hypertension generally aimed at a lower range than in the past,12 ophthalmologists can now provide their colleagues in medicine with “individualized goals” for certain patients to ensure that the target OPPs are achieved.
- OCT-A research can provide greater insight into how adjusting bedtime BP medications can affect or reduce the risks of nocturnal hypotension.
Because OCT-A is still a relatively new modality, literature does not yet exist elucidating how OCT-A patterns and BP measures may affect glaucomatous progression. Further research is needed to guide practical measures on the clinical applications of OCT-A and how regulating OPP may help patients. Future studies showing how adjustments in ocular perfusion slow disease progression could someday lead to shifts in glaucoma treatment strategies. While eye care providers await trials to assess the true effects of adjusting perfusion, they can begin to consider a new type of “pressure” in glaucoma care.
1. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701-713.
2. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714-720.
3. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:10:1268-1279.
4. Anderson DR. Collaborative Normal Tension Glaucoma Study. Curr Opin Ophthalmol. 2003;14(2):86-90.
5. Tsai JC. Influencing ocular blood flow in glaucoma patients: the cardiovascular system and healthy lifestyle choices. Can J Ophthalmol. 2008;43(3):347-350.
6. Grieshaber MC, Flammer J. Blood flow in glaucoma. Curr Opin Ophthalmol. 2005;16:79-83.
7. Flammer J, Orgül S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359-393.
8. Wang Y, Bower BA, Izatt JA, et al. Retinal blood flow measurement by circumpapillary Fourier domain Doppler optical coherence tomography. J Biomed Opt. 2008;13:064003.
9. Jia Y, Wei E, Wang X, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014;121(7):1322-1332.
10. Liu L, Jia Y, Takusagawa HL, et al. Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol. 2015;133(9):1045-1052.
11. Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Invest Ophthalmol Vis Sci. 2016;57(9):OCT451-459.
12. SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Eng J Med. 2015;373(22):2103-2116.