Sponsored by TearLab
This article highlights key topics discussed during a symposium on the TearLab Osmolarity System.
I have used the TearLab Osmolarity System for 7 years. When I first got the test, I was excited to use it, but I made assumptions about dry eye disease (DED). I thought a diagnosis was determined by patients’ symptoms and slit-lamp examinations. When the osmolarity measurements that I obtained were clinically confusing, I thought the device was malfunctioning. I thought I was right, and the device was wrong. Then I began learning about eye-to-eye variability in DED. DED is not a simple disease. The osmolarity test opened my eyes to what a mistake it is to diagnose every symptom of scratchiness, irritation, and dryness as DED and simply prescribe artificial tears. It was my “aha” moment.
—Christopher E. Starr, MD, moderator
Learning to Use the Device
Christopher E. Starr, MD: Let us discuss how you began with TearLab and any growing pains that you experienced.
Guy Smith, MD: The regulation of the ocular surface is complicated, but the management of it does not need to be. The cause of patients’ ocular surface problems is likely inflammation or abnormal osmolarity. You can test patients’ tear break-up time (TBUT), but it is not a reliable test because it depends on how it is administered. I think you should believe patients’ symptoms and not think of them as complainers. The next step is to measure their osmolarity. If they have abnormal osmolarity or a difference of more than 8 mOsm/L between the two eyes, then address it. If symptoms persist, then address the inflammation. Sometimes, both can be addressed at the same time.
Omid Kermani, MD: I can tell you this story from the point of view of an eye surgeon. I became an eye surgeon because I do not like to talk too much to my patients. If I wanted to talk to my patients, I would have become a psychiatrist.
If I did everything right, including preoperative calculations, the outcome should be perfect. I think I have done a perfect job, and my team has done a perfect job. Yet, the patient complains. Then, we start considering all aspects, including the differential diagnosis of tear film deficiency. It is a big catalog of options. This is where the TearLab device is helpful, because it has changed our approach to the problem. In the past, if the postoperative outcomes were not positive, then you had to talk to patients over and over again.
I think the approach has changed in the past 10 years. We have learned that the tear film is important because it breaks the light and makes the refraction work. It is critical in terms of clinical outcomes. The paradigm has changed in that we first try to assess the tear film and then we start treatment. Dry eye is not sexy. Dry eye is like a stone in a shoe.
Erik L. Mertens, MD, FEBOphth: In my practice, I send patients who have abnormal osmolarity home with a treatment plan even if they are asymptomatic. At their 1-month follow-up, I retest them. I take IOL measurements only when their osmolarity is normal, because then I am sure that there will not be an error in my calculations.
I began using the TearLab device 2 years ago, but it was confusing. What do I do if I find symptomatic or asymptomatic eyes with abnormal osmolarity? So, I returned the device. Then, TearLab taught me how to use the device. It is not the symptoms that determine if a patient has abnormal osmolarity, and asymptomatic patients do not necessarily have normal osmolarity.
When it comes to IOL power calculations, especially with toric IOLs, this can become a key issue. The accuracy of corneal radius measurements depends on the tear film quality. Placido-based diagnostic devices usually reveal tear film deficiency by poor reflectance. With diagnostic devices that take only two or four points as a reference, it can be difficult to determine tear film deficiency. The mismatch of values taken with different diagnostic machines is a strong hint that the tear film may not be in good condition.
When patients come to my office for their initial visit, the first thing I do is a TearLab measurement. I have a fixed fee for these pre-examinations. I always tell patients that their tear film is important. How do I make the patient understand how important it is? I have found the following explanation to resonate with patients: If you are driving a car in the rain and your windshield wipers are bad, then stripes will form on the window. How is your vision? Is it blurry with halos and starbursts? It is not good because of your tear film.
If the patient has abnormal osmolarity, then I will not do other tests because they are useless. I have them start treatment and return in 1 month.
Potential Corneal Damage
Dr. Kermani: Is the abnormal osmolarity damaging anything at the corneal level?
Dr. Starr: Yes. Abnormal osmolarity is proinflammatory. It damages epithelial cells. It has long-term effects on corneal nerves, which is why it gets complicated in terms of symptoms and signs.
Luca Vigo, MD: Should we tell patients with abnormal osmolarity that they need treatment even if they are asymptomatic because the abnormal osmolarity may damage their nerve sensors?
Dr. Starr: I tell patients with early, mildly symptomatic disease with abnormal osmolarity that it will likely worsen with time if not treated appropriately now. That worsening could damage the nerves and other structures over time.
Dr. Kermani: In the past 6 months, I have learned that abnormal osmolarity increases the sensitivity of the cornea. Therefore, I have changed my attitude toward patients with abnormal osmolarity. I now measure the osmolarity of all patients who receive LASIK and premium IOLs, such as multifocals. If they have abnormal osmolarity—regardless of the medications they take or how long they have worn contact lenses—I try to treat it.
Dr. Vigo: I make a comparison to glaucoma. For patients with high IOP, we tell them that it is not good for their eyes even though they do not have any visual symptoms. They need to start therapy to manage the issue. I need more evidence about this, but if we are agreeing on the fact that abnormal osmolarity will damage the corneal cells or nerves, then we should talk to patients early. They may not have signs now, but they may have signs in the future. Ethically, we should inform patients that they need treatment even if they do not have symptoms.
Abnormal Osmolarity and Surgical Outcomes
Dr. Kermani: In Cologne, Germany, patients often present with red eyes and other symptoms, but they have normal osmolarity. In summer, it is hot and humid, and in winter, it is smoggy. These conditions have a strong effect on patients’ tear films. Is it allergies, inflammation, stress, or sleeplessness? Many factors affect patients’ tear films. If patients have abnormal osmolarity, then surgery is not an option. The TearLab Osmolarity System is a helpful differential diagnosis tool. If a patient is asymptomatic with abnormal osmolarity, then I address it.
Audience: After you treat refractive patients’ abnormal osmolarity, do you take biometry and topography measurements again at their 6-week follow-up?
Dr. Kermani: It depends on the values given by the osmolarity test. I retake measurements if it is a severe case of abnormal osmolarity. I would not recommend surgery, and I would refer the patient to supportive therapy. These patients are usually happy because they are now aware of the problem. Tear film deficiency and DED are all over the Internet. I address the problem with objective testing, which patients understand and find helpful.
Audience: How did you define severe?
Dr. Starr: In general, I consider the patient to have DED if tear osmolarity is 308 mOsm/L or above, in either eye, or there is a difference between the two eyes of 8 mOsm/L or more. Moderate DED is from 320 to 330 mOsm/L, and above 330 mOsm/L is severe DED.
Audience: Are there published or peer-reviewed data showing that abnormal osmolarity needs to be addressed before performing LASIK or refractive procedures?
Dr. Starr: There is peer-reviewed literature that does show that abnormal osmolarity should be addressed before cataract or refractive surgery. For instance, Epitropoulos et al1 determined that abnormal osmolarity found preoperatively in cataract patients correlated to an IOL calculation error of anywhere from 0.50 to 1.00 D. The patients with normal osmolarity had no significant variation. If abnormal osmolarity is not addressed preoperatively, then an IOL calculation error may occur.
Many surgeons wonder whether or not to perform surgery in patients with abnormal osmolarity. I tell patients who have ocular surface disease, DED, and abnormal osmolarity preoperatively that I cannot choose with accuracy the lens that will give them the best visual outcome without wearing glasses. I can treat their DED aggressively. After the ocular surface is optimized, the topography, keratometry (K) readings, and IOL power calculation will be more accurate. If you spend extra time initially, you will save yourself a lot of time afterward. Patients and doctors do not want a refractive surprise or to spend an enormous amount of chair time postoperatively discussing piggyback lenses, IOL exchanges, or LASIK touch-ups. If it is diagnosed postoperatively, DED is viewed as a surgical complication. If you diagnose and address it preoperatively, you are a hero.
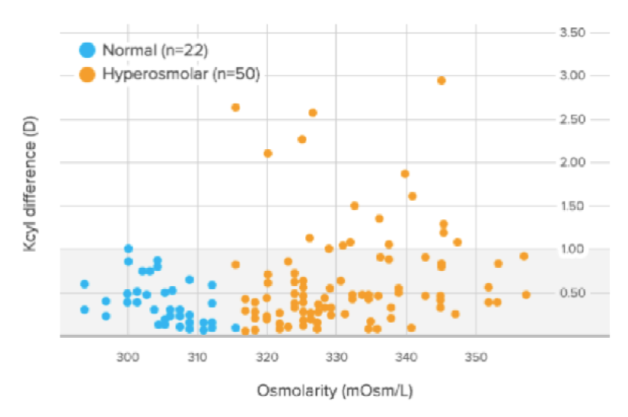
Figure 1 | In the Epitropoulos study, 17% of eyes with abnormal osmolarity had >1.00 D of difference in K cylinder values between two presurgical visits, and 10% of eyes with abnormal osmolarity had >0.50 D of change in calculated IOL power (based on average K).
Dr. Smith: In the Epitropoulos study,1 10% of patients experienced an IOL refractive surprise of more than 0.50 D spherical equivalent (Figure 1). The highest refractive surprise was 5.50 D, which is difficult to explain to a patient.
Differential Diagnosis
Dr. Starr: I presented a poster at the 2016 Tear Film & Ocular Surface Society conference about a prospective study of 50 patients who were symptomatic with normal osmolarity.2 The diagnosis for the vast majority of those patients was either allergic conjunctivitis or anterior blepharitis (Figure 2). Itching is not just allergies, it can be DED too. Dryness is not just DED, it can also be allergies or other things. If you are relying on symptoms predominantly, you are going to miss the correct diagnosis frequently. Other common diagnoses were conjunctivochalasis, epithelial basement membrane dystrophy, keratoneuralgia, and contact lens intolerance. You can determine if a patient has any of these conditions rather than DED because you are driven to them by the normal osmolarity. Without the normal osmolarity test, many of these patients may have been misdiagnosed and mistreated as simple DED.
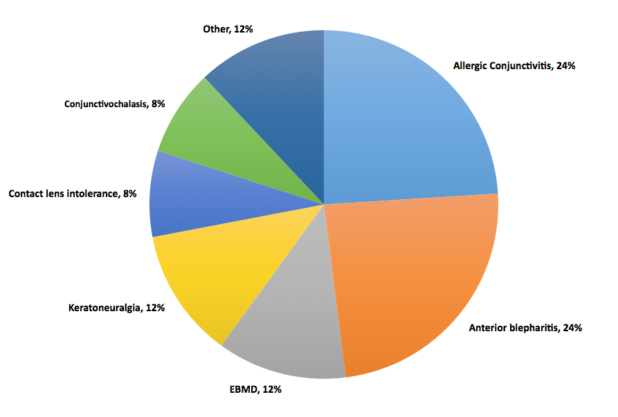
Figure 2 | Diagnosis of patients who were symptomatic with normal osmolarity.
What if a patient has minimal symptoms or is asymptomatic but has severely abnormal osmolarity? Is that a sign of early disease that will most likely progress? I am sure you have all seen in your practices that DED gets worse if not treated.
The Progression of Ocular Findings (PROOF) study, led by Peter McDonnell, MD, from the Wilmer Eye Institute,3 was the first prospective, controlled study on the natural history of DED. One of the interesting things about the baseline cohort in that study is that both groups (dry eye group and nondry eye group) included patients over the age of 60 with a baseline mean Snellen acuity of 20/20. However, the group that had dry eye complained of poor quality and blurred vision (58.5% vs 13.7%.). This shows how important the tear film is for vision. These patients were 20/20, but they were not happy with their vision. They experienced fluctuations in vision, blurring, clouds, ghosting, and starbursts. This is because the ocular surface was not treated. One of the findings in the PROOF study was that the dry eye group had qualitatively worse vision, all related to the tear film. There are studies that show significant reductions in retinal image quality in patients with DED.4
Dr. Kermani: There is another simple aspect that we sometimes forget about. As refractive surgeons we sometimes think, why do patients complain when the refraction is perfect? As Dr. Starr mentioned, they have blurred vision that fluctuates, among other visual symptoms. I think it is because we take away their glasses. Without glasses, their eyes have no physical protection from ultraviolet or artificial light, ozone, smog, and the reflection of screens. This increases the stress of the tear film significantly and leads to tear film decompensation.
Dr. Starr: I never thought about that before. But now that you said it, it makes perfect sense. There are so many reasons why eyes get dryer after any kind of intervention, whether it is cataract surgery, limbal relaxing incision, LASIK, PRK, or others. They can be affected by the incisions, ablations, drops and preservatives, povidone iodine, exposure from the speculum, light toxicity from the microscope, suction, etc. All of those things can have a negative impact on the ocular surface.
Dr. Vigo: Honestly, I hate the tear film, because it causes the most frequent complaints from patients after cataract surgery. Patients say that every time they blink, it gets better, and every time they blink, it gets worse. It is one of the major issues in my practice. Are there guidelines on how to deal with these patients who have abnormal osmolarity? When I follow-up with patients 1 month postoperatively, they have severely abnormal osmolarity. What do I do from a practical point of view?
Dr. Starr: The patient is not on medication and has 20/20 vision, but is not satisfied with the quality of his or her vision. This patient has rapid TBUT, staining, and a low tear lake. With DED, it is important to determine whether it is evaporative, aqueous deficient, or a mix. It may not even be DED. If it is primarily evaporative, as is often the case, you can treat it with lipid-based artificial tears to stabilize the lipid. You can treat the meibomian glands with warm compresses, perform lid hygiene, and increase the patient’s intake of omega-3 fatty acids through supplementation. A paper was recently published about the value of omega-3s in these patients (Figure 3).5 Procedures such as intense pulsed light therapy, BlephEx (RySurg), and LipiFlow (TearScience), which are all available in the United States, are helpful as well and may work faster than conventional treatments.
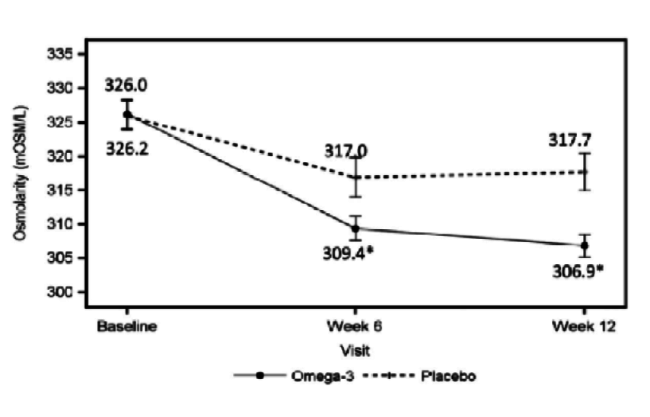
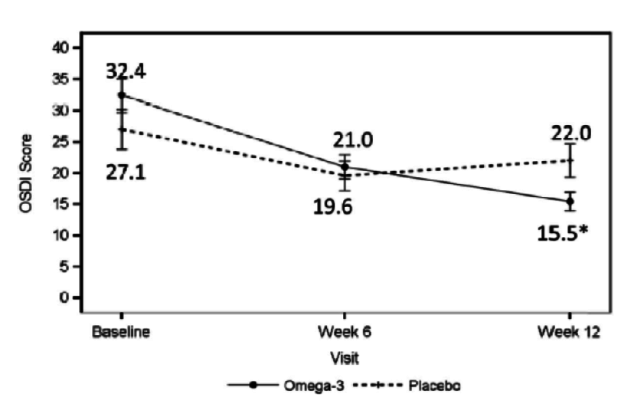
Figure 3 | The omega-3 treatment group had a significant decrease in tear film osmolarity at 6 weeks (P = .042) and 12 weeks (P = .004) after initiation of treatment.
If it is primarily aqueous deficient DED, then you want to replenish the tears with artificial tears and punctal plugs, and perhaps prescribe anti-inflammatories like steroids, cyclosporine, or lifitegrast. There are medications such as secretagogues that are not available in the United States but are available in other parts of the world. With those patients who are unhappy postoperatively, I tend to use a lot of treatments at once, because I want them to get better quickly.
Dr. Vigo: Do you think that the osmolarity measurement will help us determine the course of treatment?
Dr. Starr: Absolutely. The higher the osmolarity number, the more aggressive you want to be with your treatment in order to reverse that number and the disease as quickly as possible. The osmolarity measurement is not accurate if patients have used drops within 2 hours of testing, so be on the look out for this in the postoperative period when patients tend to be on multiple drops.
Dr. Smith: I have a patient with severe ocular surface disease, secondary ocular cicatricial pemphigoid, and glaucoma, but with normal osmolarity. If you see superficial punctate keratitis in the paplebral aperture, then it is likely evaporative DED. If you see superficial punctate keratitis under the eyelid, then it is likely to be inflammatory. I increased this patient’s lubricants and focused on anti-inflammatories. With these patients who have complex ocular surface disease, it is important to determine if you are treating abnormal osmolarity or inflammation. If they have abnormal osmolarity, I will increase their topical lubricant.
Audience: It depends on whether it is DED with abnormal osmolarity or normal osmolarity.
Dr. Smith: If patients have allergic conjunctivitis, I would prescribe a thin lubricant such as preservative-free hypromellose eye drops, because I do not want antigens on their ocular surface. If patients have severe Sjogren syndrome, then hyaluronic acid works well. These patients do well with 0.1%, they do better with 0.2%, and they do even better with 0.4%. It is important to use preservative-free drops, but not everyone has access to preservative-free medication.
Billing the Patient
Audience: How do you bill the patient?
Dr. Starr: Billing the patient depends on where you practice. In the United States, we have a reimbursement code, but in order to obtain reimbursement from the insurance companies for an osmolarity test, patients have to present with symptoms.
Dr. Smith: In the United Kingdom, each hospital negotiates a tariff with their local commissioning group. Some commissioning groups will pay for TearLab as an extra test, others ask for it to be bundled into the outpatient tariff.
Osmolarity 101
Dr. Starr: We are lucky to have here with us Benjamin Sullivan, PhD, the inventor of the TearLab device.
Benjamin Sullivan, PhD: I will discuss the ocular surface itself. Most of you have seen the epithelium before, but, on the nanometer scale, the microplicae and inside of it, the glycocalyx, become visible, which are basically sugars that hold onto water. These structures, which lower the surface area of contact so that there is less friction from blinking, are compromised in an ocular surface with abnormal osmolarity. The ocular surface cells progress from columnar and closely packed cells in normal osmolarity, to becoming desquamated in a moderate osmolarity, to dying and sloughing off in severe osmolarity.
Audience: How high is the osmolarity?
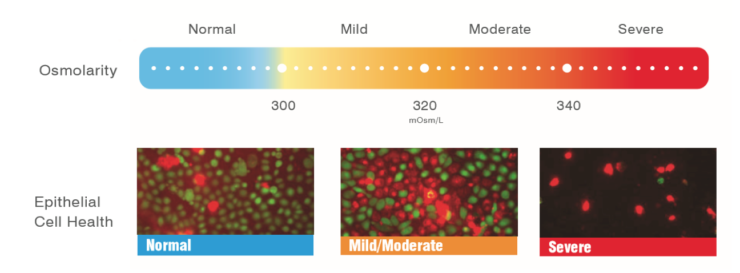
Figure 4 | When the osmolarity is normal, the cells are vibrant, green, and happy. At 338 mOsm/L, the cells are red and blebbing, and at 338 mOsm/L, the cells are dying en masse.
Mr. Sullivan: Green is alive. Red is dead (Figure 4). When the osmolarity is normal, the cells are vibrant, green, and happy. At 338 mOsm/L, the cells are red and blebbing, which means they are liquefying their contents and undergoing apoptosis. The cells are dying en masse at 338 mOsm/L. At 600 mOsm/L, all the cells are dead. So, with normal osmolarity, the cells are happy, and with abnormal osmolarity, they start dying.
Nerves also start dying. Abnormal osmolarity causes the structure and the function of the nerves to change. The nerves become sensitized early in the disease, and then they die off as the disease progresses.6,7 You cannot know if a patient is symptomatic or asymptomatic by simply looking at them, because the nerves are not functioning properly. As Dr. Starr mentioned, we are learning a lot more about neuropathic pain. In addition to aqueous and evaporative, some are suggesting adding a third neuropathic arm to the main classification of DED.
In a study by DeMill and colleagues evaluating diabetic patients with peripheral neuropathy,8 the patients had many severe signs of DED, but without the concomitant increase in symptoms. In the DED population, patients with diabetes are common, but they can present as asymptomatic. Would you do open-heart surgery on a patient with 104º fever? No, you would lower the patient’s temperature before performing surgery. Osmolarity is like your body temperature: The higher the osmolarity, the less healthy the ocular surface. It is less capable of healing itself.
For example, rabbits that have had their eyes held open and exposed to air for several hours have obvious staining after 4 hours. Do you think after 1 hour that these rabbits have problems with their ocular surface? After an hour of holding my eye open, I would probably be very unhappy. Osmolarity is useful because it is an early-stage marker for DED. The osmolarity in the rabbits’ eyes was likely elevated to greater than 1,000 mOsm/L quickly in that study. As a result, there was a massive infiltrate of inflammatory cells into the conjunctiva and up to the limbus because of that stimulus. Interestingly, the authors found no inflammatory cells at the superficial or basal epithelial layers of the cornea during the observational period.9
Abnormal osmolarity induces a strong proinflammatory response. Strielein showed that if the nerves are severed, then the cornea loses its immune privilege and inflammatory cells can rush in.10 During transplants and refractive surgery, the cornea’s immune privilege is likely lost when the nerves are cut. It is likely that, in this exposure model, the abnormal osmolarity did not have time to compromise the nerves in the cornea. Of note, after the rabbits closed their eyes, all of the inflammatory cells in the rabbits’ eyes disappeared 24 hours later. Why? The osmolarity was reduced. If you remove the toxic stimulus—the abnormal osmolarity—the ocular surface can normalize. In the cornea, inflammation and abnormal osmolarity are two different processes that should be treated independently.
Audience: What is that toxic stimulus?
Sullivan: Abnormal osmolarity.
Audience: The rabbits had only abnormal osmolarity?
Mr. Sullivan: Yes, the rabbits just had their eyes kept open.
Audience: How do you prove that cytokines are not present?
Mr. Sullivan: That is a good question. Cytokines were likely present, but the cause of those cytokines was the exposure and resultant abnormal osmolarity. DED is a complex disease. The anti-inflammatories that we prescribe are powerful—they downregulate cytokines and deactivate T-cells. Yet why does it require several weeks to months for the benefits of anti-inflammatories to be realized by many patients? If you have both anti-inflammatory signals mixed with strongly proinflammatory signals (eg, high osmolarity) the effects of anti-inflammatory medications alone are likely dampened. I believe that if you could normalize the microenvironment of the ocular surface in combination with an anti-inflammatory compound, you would observe far faster resolution of symptoms. As in the exposure model,10 once the abnormal osmolarity was removed, the inflammation resolved overnight. Therefore, I agree with Dr. Starr that osmolarity and inflammation have to be managed at the same time.
Audience: We would like to measure matrix metalloproteinase 9, but it is not that simple.
Sullivan: We would, too. There are many factors that compromise the ocular surface that we would like to measure, including matrix metalloproteinase 9. Also, we see the same problems with benzalkonium chloride exposure. When patients are transitioned from preserved to nonpreserved glaucoma drops, their osmolarity is reduced and subjective complaints are reduced from 100% to 36%.11 If the toxic preservative is removed, the ocular surface can quickly return to normal. Data from the Epitropoulos study1 showed that when you have an unstable ocular surface where the tear film is damaging cells, the eye’s osmolarity and smoothness may change from blink to blink.1 The TBUT will be 10 seconds, and then it will be 3 seconds. In this scenario, it is difficult to gather topography measurements.
Dr. Mertens: I am a surgeon, not a scientist like you. We all know that K readings is the most important factor to determine the correct IOL power. If you are off slightly, it has a huge impact on your power calculations. It was indeed the work of Epitropoulos1 that showed that patients with abnormal osmolarity had a statistically significant difference in IOL power calculations. In 17% of the eyes with abnormal osmolarity, there was a difference of more than 1.00 D in IOL power calculations. We often discuss DED, but not the impact it has on our surgical precision. We would like to have a certain percentage of eyes within ±0.50 D of intended correction, but most of us do not give enough attention to patients’ abnormal osmolarity.
The Future of TearLab
Mr. Sullivan: We have spent many years developing a second-generation TearLab device that can actually measure inflammatory mediators and other proteins in about 1.5 to 2 minutes. Most testing to get inflammatory information takes about 15 minutes. We can multiplex as well. So far, we have measured up to three proteins and osmolarity at the same time. We are excited to bring it to Europe first. We do not yet know what exactly will be measured yet, because we have a menu of 10 to 12 proteins, but we need to validate. We are curious to see different kinds of inflammation in the different types of DED. We want to know how long it takes to treat. We plan to launch it at the 2017 annual meeting of the European Society of Cataract and Refractive Surgeons (ESCRS). In the meantime, we will be validating different clinical markers and conducting research.
Dr. Mertens: You mentioned inflammatory markers. Are allergies the third group of indicators that you want to measure?
Mr. Sullivan: Yes. We have allergy markers. We have potential glaucoma markers. There are reports of keratoconic markers in the tear film. The vision behind TearLab is not to just be a DED-focused company. It is to bring in vitro diagnostics to ophthalmology. If we have molecular-level information about the cause of a disease, we can quickly get the right therapy to the right person. That is the larger vision of TearLab.
Dr. Mertens: I am looking forward to having this device in my clinic.
1. Epitropoulos AT, Matossian C, Berdy GJ, et al. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41(8):1672-1677.
2. Brissette A, Bohm K, Drinkwater O, Starr C. The diagnostic utility of a normal tear osmolarity test in patients with dry eye disease-like symptoms: A prospective analysis. Poster presented at: 2016 Tear Film & Ocular Surface Society conference; Sept. 7-10, 2016; Montpellier, France.
3. McDonnell P, Pflugfelder S, Schiffman R, et al. Progression of ocular findings (PROOF) study of the natural history of dry eye: Study design and baseline patient characteristics. Invest Ophthalmol Vis Sci. 2013;54(15):4338.
4. Liu H, Thibos L, Begley CG, Bradley A. Measurement of the time course of optical quality and visual deterioration during tear break-up. Invest Ophthalmol Vis Sci. 2010;51(6):3318-3326.
5. Epitropoulos A, Donnenfeld E, et al. Effect of oral re-esterified omega-3 nutritional supplementation on dry eyes. Cornea. 2016;35(9):1185-1191.
6. Parra A, Gonzalez-Gonzalez O, Gallar J, Belmonte C. Tear fluid hyperosmolarity increases nerve impulse activity of cold thermoreceptor endings of the cornea. Pain. 2014;155(8):1481-1491.
7. Hirata H, Mizerska K, Marfurt CF, Rosenblatt MI. Hyperosmolar tears induce functional and structural alterations of corneal nerves: Electrophysiological and anatomical evidence toward neurotoxicity. Invest Ophthalmol Vis Sci. 2015;56(13):8125-8140.
8. DeMill DL, Hussain M, Pop-Busui R, Shtein RM. Ocular surface disease in patients with diabetic peripheral neuropathy [published online ahead of print October 23, 2015]. Br J Ophthalmol. doi: 10.1136/bjophthalmol-2015-307369.
9. Lai CT, Yao WC, Lin SY, et al. Changes of ocular surface and the inflammatory response in a rabbit model of short-term exposure keratopathy. PLoS One. 2015;10(9):e0137186.
10. Streilein JW, Okamoto S, Sano Y, Taylor AW. Neural control of ocular immune privilege. Ann N Y Acad Sci. 2000;917:297-306.
11. Janulevičienė I, Derkač I, Grybauskiene L, et al. Effects of preservative-free tafluprost on tear film osmolarity, tolerability, and intraocular pressure in previously treated patients with open-angle glaucoma. Clin Ophthalmol. 2012;6:103-109.