—Case prepared by Alan N. Carlson, MD.
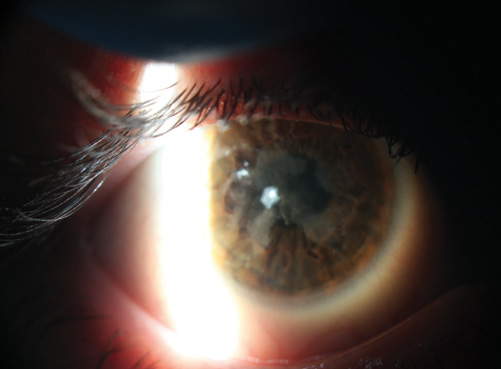
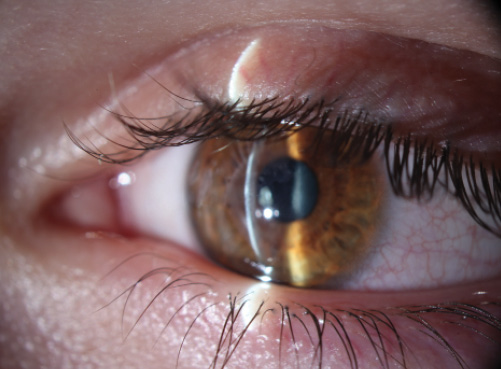
Figure. Anterior corneal scarring affects vision by causing opacification and surface irregularity. Unlike in Salzmann nodular degeneration, the anterior stroma is involved at a depth of 50 to 75 μm.

ZAINA AL-MOHTASEB, MD
The appearance of the multiple white, elevated subepithelial corneal nodules are consistent with Salzmann nodular degeneration. The central location of the nodules with accompanying opacification and irregular astigmatism has decreased this patient’s vision. Preoperatively, I would use Placido ring topography to evaluate the irregularity of the corneal surface (surface regularity index and surface asymmetry index) and Scheimpflug imaging to assess corneal pachymetry. Anterior segment optical coherence tomography (OCT) could also measure the depth of the nodules to help guide the depth of treatment.
I would counsel the patient on the risk of scarring, recurrence, and amblyopia, because the condition has been present since birth. Unlike the typical hyperopic shift after PTK, a myopic shift has been reported after Salzmann nodular degeneration excision (as a result of an asymmetric pooling of the tear film between the nodules). In this case, the nodules are central, and we cannot be certain of the postoperative refraction.
My primary surgical goal would be to improve surface regularity, not to fully remove the opacity. After removing the epithelium, I would grasp the nodules with a toothed forceps and perform a planar dissection with a blunt spatula. Some nodules will easily separate from Bowman membrane, whereas others may result in multiple breaks in this layer of the cornea. I would perform PTK using repeated applications of masking fluid until the surface of the cornea became smooth (nodules that do not separate easily generally require a deeper ablation to smooth the corneal irregularities). I would start with a small spot size (2-3 mm) to treat the elevated lesions and then switch to a larger spot size (6.5 mm) to treat the whole area. Once the surface was smooth, I would place a 9-mm sponge soaked in mitomycin C 0.02% for 30 seconds on the surface to decrease the risk of recurrence and scarring, then irrigate with balanced salt solution. I would place a contact lens at the end of the case and prescribe postoperative topical antibiotics and steroids.

MARK S. GOROVOY, MD
The findings of congenital, bilateral scarring without a confirmatory cause is a concern, because we physicians do best with known diagnoses and established, evidence-based medicine. Unfortunately, not all clinical findings fit into a recognizable disease state, as seems to be the case here. What is known is that the scarring is bilateral and stable without any inflammation. Because clinically it most resembles Salzmann nodular degeneration, I would recommend treating it accordingly.
I agree that superficial keratectomy is indicated. Corneal OCT would define the depth of the scarring. As with Salzmann nodular degeneration, I would attempt a manual keratectomy at the slit lamp using toothed forceps. During that procedure, it would be easy to tell if the scars peeled off smoothly, indicating a successful optical outcome, or if the scars were so deep as to preclude a smooth surface and good outcome. In the latter instance, I agree that the next step would be a PTK with masking agents, and if that were unsuccessful, then an anterior lamellar graft should be done.
I would only work on one eye so as to determine the best treatment for the second eye, because they will behave the same. I would not jump to PTK before attempting the manual keratectomy. I base this decision on my experience with many successful manual keratectomies, despite some mild residual scarring that, in this patient, might be insignificant because of a component of amblyopia present since birth.

PARAG A. MAJMUDAR, MD
The etiology of anterior corneal opacities can often be elusive. Genetic, traumatic, infectious, and idiopathic sources need to be considered. If truly present since birth, this patient’s scarring may be the result of an in utero insult, and in that case, there may be a significant degree of amblyopia in both eyes.
Corneal topography, which was not available, might shed some light on the degree of irregularity. The assumption is that a rigid gas permeable (RGP) contact lens was used to mask corneal irregularity and resulted in no improvement in BCVA. As such, it is likely that the recorded acuities of 20/40 OD and 20/50 OS may be the best that are achievable. This does not preclude the possibility of surgical intervention, however, because regularization of the corneal surface might improve the quality of vision if not the acuity. Many times, we refractive surgeons dwell on the Snellen number and forget that visual quality is truly independent of the Snellen fraction. For example, in the presence of irregular astigmatism, a patient may yet be able to read the 20/20 Snellen line, but multiple images may degrade the quality of that image and be visually detrimental. In this case, even though the patient can read 20/20, it may not result in “20/happy.”
With that background in mind, I would counsel the patient regarding expectations. Specifically, I would advise him that amblyopia is likely a factor in both eyes and that the goal of intervention would be to normalize the surface and reduce irregular astigmatism so that he could benefit from RGP or hybrid contact lenses postoperatively.
Presurgical considerations would be to evaluate the corneal thickness using a Scheimpflug device or OCT. I would perform unilateral (sequential) surgery and stage the procedure in each eye with the primary goal of removing the Salzmann-like nodules. This can often be performed in an in-office minor procedure room under topical anesthesia. For young patients, or those less likely to cooperate, intravenous sedation in the setting of a surgical suite may be more appropriate. In addition, although Salzmann lesions are often easy to remove, because there is an identifiable plane of dissection above Bowman membrane, if the location is close to the limbus, there may be bleeding, requiring cautery for hemostasis.
I would not address anterior stromal scarring at this time. Often, the epithelium will act as a masking agent. Once the surface had regularized for several weeks, I would reassess corneal topography and BCVA. It might be beneficial to repeat an RGP contact lens overrefraction at that time as well. If the topography were more normal and the patient were symptomatically better, the same procedure could be performed in the fellow eye.
The decision to proceed to PTK would be based on the level of improvement in symptoms and acuity as well as the extent/depth of stromal scarring. PTK is typically not recommended if the opacity is located at a depth greater than 100 µm. The use of masking agents such as the patient’s own epithelium or carboxymethylcellulose allows the laser to remove the “peaks” but not the “valleys, ensuring a new, more uniform surface rather than a copy of the prior surface irregularity.

WHAT I DID: ALAN N. CARLSON, MD
The panel’s responses are remarkably consistent and match my colleagues’ and my approach to this patient. Although lamellar keratectomy and lamellar keratoplasty were considerations, we opted for a more conservative initial approach. We treated the left eye first with keratectomy under the Star S4 laser (Abbott) in hopes that the pathology would peel like a Salzmann nodule. This was not the case. Instead, the pathology was adherent and involved the anterior stroma too deeply for debridement alone.
We programmed the Star S4 laser for PTK, circle mode, and used a masking agent (Tears Naturale II; Alcon). This technique involved coating the corneal surface, peripherally wicking away the excess, and then waiting until enough dissipation and evaporation occurred for the corneal “peaks” to begin to appear. PTK was intermittently applied to these peaks while the masking agent continued to redistribute itself, covering the “valleys.” We periodically reapplied the masking agent. Although the laser was programmed for 200 µm of PTK, this technique using a masking agent made it so that treatment had only minimal if any impact on the valleys within the pathology. Pausing periodically for evaluation using the slit-lamp biomicroscope eventually showed a satisfactory outcome, after which we applied mitomycin C for 45 seconds.
In addition to protecting his eyes from ultraviolet light, we recommended a longer course of topical corticosteroids than our routine taper over a 2-month period after PTK for recurrent corneal erosion or Salzmann nodular degeneration. We were pleasantly surprised that our patient went from a poor-quality BCVA of 20/50 with a high degree of regular and irregular astigmatism to a remarkable UCVA of 20/30 at 3.5 weeks postoperatively. His quality of vision while healing continues to improve, and we have scheduled his second eye for surgery. n
Dr. Carlson would like to acknowledge the efforts of Tina M. Mazzurco, COA, not only for the care of this patient but also her contributions to this article.
Section Editor Alan N. Carlson, MD
• professor of ophthalmology and vice chair, departmental development, Duke Eye Center, Durham, North Carolina
• (919) 684-5769; carls009@mc.duke.edu; alancarlsonmd.com
• financial interest: none acknowledged
Section Editor Stephen Coleman, MD
• director of Coleman Vision, Albuquerque, New Mexico
Section Editor Karl G. Stonecipher, MD
• clinical associate professor of ophthalmology, University of North Carolina, Chapel Hill
• director of refractive surgery, TLC in Greensboro, North Carolina
Section Editor William F. Wiley, MD
• private practice at Cleveland Eye Clinic, Cleveland, Ohio
Zaina Al-Mohtaseb, MD
• assistant professor of ophthalmology and associate residency program director, Baylor College of Medicine, Houston
• cornea, cataract, and refractive surgery, Baylor College of Medicine, Houston
• (713) 798-5143; zaina@bcm.edu; Twitter @zaina1225
Mark S. Gorovoy, MD
• private practice with Gorovoy MD Eye Specialists, Fort Myers, Florida
• (239) 939-1444; mgorovoy@gorovoyeye.com
Parag A. Majmudar, MD
• associate professor, Cornea Service, Rush University Medical Center, Chicago Cornea Consultants, Chicago
• (847) 822-5900; pamajmudar@chicagocornea.com


