
As a medical subspecialty, glaucoma is unique in several ways. One of the less flattering particularities of the field is our inability to characterize the disease’s only treatable risk factor better than by obtaining a mere snapshot of IOP. Furthermore, our so-called gold standard (Goldmann applanation tonometry [GAT]) is anything but golden; it is one of the oldest technologies in medicine that has remained unchanged (GAT was invented before most readers of CRST were born).
AT A GLANCE
• A device capable of measuring IOP continuously over the 24-hour period has been one of the main unmet needs in ophthalmology for decades.
• The FDA’s 2016 approval of the Triggerfish contact lens sensor opens the path to its clinical use. In addition, implantable IOP sensors recently entered clinical trials.
• These devices promise to fundamentally change the way in which glaucoma is managed.
The main limitation of GAT and other tonometry techniques is the static nature of their measurements. It is widely accepted that IOP is a dynamic parameter with an individual circadian rhythm and (sometimes significant) spontaneous changes. There is strong evidence that single IOP measurements, commonly taken in seated patients and during normal clinic hours, neither reflect the true range of an individual’s IOP nor the peak IOP. Even less well characterized are IOP fluctuations throughout the day and variations over weeks and months. Whether these constitute independent risk factors for glaucoma remains unknown.
IOP, THE SLEEPING GIANT
In the past, IOP was widely thought to be at its lowest at night, based on the knowledge that aqueous humor production is reduced by half during sleep. This prevailing dogma was called into question when research from the sleep laboratory at the University of California, San Diego, showed that both healthy individuals and glaucoma patients exhibited their peak IOP levels during the nocturnal period. The physiological mechanisms were initially unknown, and part of the glaucoma community doubted the findings. Later, however, researchers demonstrated that a combination of the supine body position, hormonal changes, and the almost complete shutdown of uveoscleral outflow are behind this phenomenon.1
The impact of the nocturnal IOP rise on glaucomatous progression is unknown. If we extrapolate data from a rat glaucoma model, it seems that elevated nocturnal IOP may be even more detrimental to axonal injury in glaucoma than high diurnal pressure.2
THE SMART CONTACT LENS
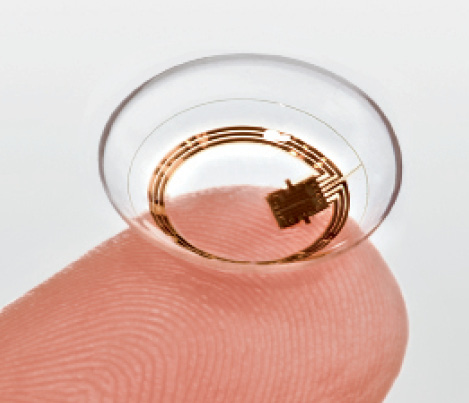
Figure. The Triggerfish contact lens sensor.
When European regulatory authorities approved the Triggerfish (Sensimed) in 2009, it became the first smart contact lens (Figure). The technology is based on the principle that the detection of ocular dimensional changes reflects alterations in IOP. The device does not measure IOP directly, and its output cannot be calibrated into mm Hg. Nevertheless, studies have shown that the contact lens’ measurements correlate strongly with tonometry. My colleagues and I compared the device to tonometry in the fellow eye of subjects housed within a sleep laboratory and found that both methods detected peak IOP at around the same time (approximately 4:00 am).3
The Triggerfish measures a composite of IOP, volume, and elasticity of ocular tissues. Changes in these parameters may reflect stress on the connective tissues of the optic nerve head, the hypothesized site of glaucomatous injury. It is therefore conceivable that output from the contact lens may reflect changes that are more relevant to glaucomatous damage than pure IOP.
THE SIGNIFICANCE OF FDA APPROVAL
On March 4, 2016, the FDA approved the Triggerfish as a class II device to detect the peak patterns of variation in IOP over a maximum period of 24 hours to identify the window of time during which to measure IOP by conventional clinical methods. The agency’s blessing was significant in several ways. Most important for the glaucoma community is that 24-hour IOP monitoring has finally moved from the academic into the clinical realm under the generic name for this new category of diurnal pattern recorder system. According to the FDA, “A diurnal pattern recorder system is a non-implantable, prescription device incorporating a telemetric sensor to detect changes in ocular dimension for monitoring diurnal patterns of IOP fluctuations.”
The FDA approval also signifies improved collaboration between the agency and industry in recent years, enabling the speedy introduction of innovation to US patients.
Finally, a Current Procedure Terminology code is in place for (albeit modest) reimbursement of 24-hour IOP monitoring (CPT III 0329T), further paving the way for technological advances to the bedside.
Watch it Now
H. Burkhard Dick, MD, PhD, discusses the European use of implantable microsensors for monitoring IOP and potential future developments.
SENSORS, INVASIVE BUT DIRECT
Direct 24-hour IOP monitoring through implantable sensors has progressed significantly in the past year as well. The Eyemate sensor (Implandata; not available in the United States) is a silicone-encapsulated intraocular device with a diameter of 11.2 mm and a thickness of 0.9 mm. The technology incorporates eight pressure-sensitive capacitors and a circular microcoil antenna. IOP measurements are performed with a reader unit held in front of the eye. The device is intended for implantation into the ciliary sulcus during cataract surgery. The first human clinical data were published recently and showed that the sensor could measure IOP continuously over periods of up to 1 year without serious adverse events.4 Larger studies and longer-term data are eagerly awaited and forthcoming.
THE ABYSS
It is a reasonable prediction that, by 2020, most tertiary glaucoma centers worldwide will offer 24-hour IOP monitoring. A looming issue, then, regards how “big IOP data” will be managed. At present, we do not know which IOP parameters are (most) relevant for glaucoma diagnosis, the prediction of progression, and the assessment of treatment success. The ability to look deeper into 24-hour IOP (and 365 days a year) will confront us with many hitherto unanswered questions about the nature of glaucoma itself. To quote Friedrich Nietzsche, “If you gaze long into an abyss, the abyss also gazes into you.”
1. Nau CB, Malihi M, McLaren JW, et al. Circadian variation of aqueous humor dynamics in older healthy adults. Invest Ophthalmol Vis Sci. 2013;54(12):7623-7629.
2. Kwong JM, Vo N, Quan A, et al. The dark phase intraocular pressure elevation and retinal ganglion cell degeneration in a rat model of experimental glaucoma. Exp Eye Res. 2013;112:21-28.
3. Liu JH, Mansouri K, Weinreb RN. Estimation of 24-hour intraocular pressure peak timing and variation using a contact lens sensor. PloS One. 2015;10(6):e0129529.
4. Koutsonas A, Walter P, Roessler G, Plange N. Implantation of a novel telemetric intraocular pressure sensor in patients with glaucoma (ARGOS Study): 1-year results. Invest Ophthalmol Vis Sci. 2015;56(2):1063-1069.
Kaweh Mansouri, MD, MPH
• consultant ophthalmologist, Glaucoma Center, Montchoisi Clinic, Swiss Vision Network, Lausanne, Switzerland
• adjoint associate professor, Department of Ophthalmology, University of Colorado School of Medicine, Denver
• +41 21 619 37 42; kawehm@yahoo.com
• financial disclosure: consultant to Sensimed



