The synthetic corneal inlay has a checkered past. Initially, interest in this technology was significant, and refractive outcomes and patient satisfaction were good. But, biocompatibility issues later led to the termination of synthetic inlays. Is it fair, however, to judge all corneal inlays on the history of one product? What about allograft and biosynthetic inlays? Do they not have more potential to succeed than their synthetic counterparts? Recent research suggests that they do.
In a multicenter European clinical trial of the TransForm corneal allograft (Allotex), patients achieved excellent refractive results, and arguably even more promising were the clinical results. In the 45 eyes of 45 patients from our center included in the study, there was not a single case of rejection or reaction.
The TransForm corneal allograft has a diameter of 2.65 mm and a thickness of approximately 22 µm. It adds 2.50 D of near power in the central pupillary optic at the spectacle plane. Each inlay is prepared into about 100 blanks from sterile human donor corneas by means of special mechanical techniques. These blanks are measured with ultrahigh-resolution OCT and shaped into lenses using a specialized excimer laser. The refractive lenticules are then stored in recombinant human serum albumin and sterilized with electron-beam irradiation. (Accelerated electrons kill bacteria and fungi, making an electron beam ideally suited for sterilization and bioburden reduction.)
CLINICAL TRIAL
Refractive results. In the European clinical trial of the TransForm inlay for the treatment of presbyopia, uncorrected distance visual acuity (UDVA) in the implanted eye was typically 1 to 2 lines less than 1.0; in other words, it was generally 0.8 (6/7.5 or 20/25) or 0.7 (6/9 or 20/30). Binocularly, patients experienced no reduction in UDVA or stereopsis. Near vision improved from an average of N15 to between N5 and N6. (N8 is typically required to read newspaper print.)
Based on these results, we concluded that the TransForm inlay may provide near vision with fewer trade-offs than would be required to achieve similar reading vision with either a monovision or blended vision approach. In my experience with the latter two approaches, N5 or N6 reading vision would require a correction of at least -1.50 or -1.75 D, which would also result in UDVA in the reading eye of usually 6/30 (20/100) or worse. There is a concomitant reduction in stereopsis at this level of correction, too.
Safety. Patients administered topical steroids for only 4 weeks, yet no corneal haze was observed. Most of the inlays were invisible under the 110-µm corneal LASIK flap. With synthetic inlays, steroids are typically tapered over the course of 3 months, yet corneas are not as quiet as in eyes that received the allograft inlays at 3 months.
Two inlays were removed from two patients. In both cases, although UDVA was 1.0 and near visual acuity was N6+, the patients could not adjust to the difference in visual acuity between their two eyes. Removing these inlays gave me confidence that this corneal allograft is truly biocompatible. I observed no signs of the implant’s removal from the moment after it was extracted from the cornea. In contrast, anyone who has removed a synthetic inlay knows that it leaves a footprint behind that may last for 6 months or longer and, in some cases, never clear completely. In the eyes of the two patients who had the allograft removed, visual performance returned to preoperative levels within days.
THE SURGICAL PROCEDURE
The surgeon or scrub nurse removes the allograft from the packaging, which contains a transport plate that looks just like a microscope slide. The lenticule is scraped off the plate and placed into balanced saline solution to wash the albumin from the inlay. We typically left the inlay in the dish of solution for at least 15 minutes.
Once the 110-µm flap has been made and the inlay is ready for placing, it is fished from the dish of balanced saline solution with an instrument shaped like a loop that has a 4- to 5-mm diameter (Figure 1). The inlay is suspended in the layer of solution that forms from surface tension within the loop.
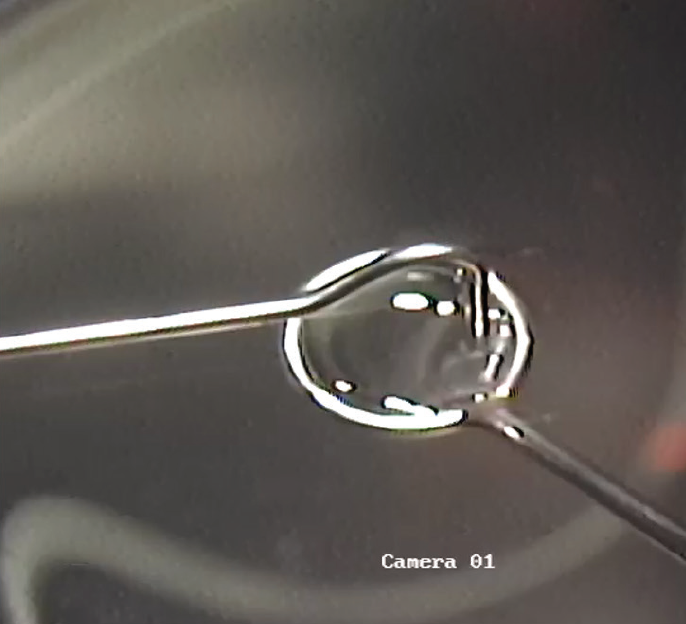
Figure 1. The TransForm inlay is fished from a dish of balanced saline solution.
The inlay is placed on the stromal surface over the light-constricted pupil. A Weck-Cel sponge (Beaver-Visitec International) is placed at the external surface of the loop. The sponge removes balanced saline solution from within the loop, which allows the inlay to settle on the corneal surface (Figure 2).
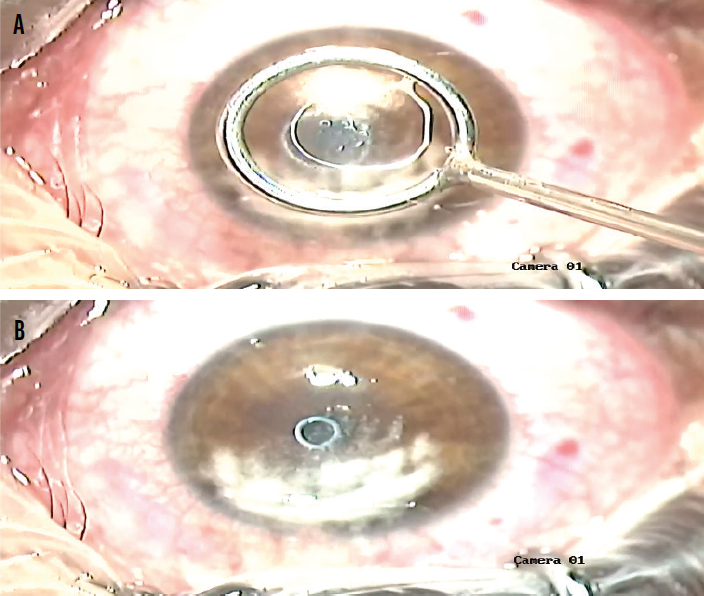
Figure 2. The tool containing the lenticule is centered over the pupil (A), and the lenticule is transferred from the tool to the eye (B).
Figures courtesy of Allotex
While the corneal inlay is wet or moist, it can be manipulated with surgical instruments to flatten its edges and refine its position relative to the pupil. Once the inlay dries, it settles well and becomes nearly impossible to move. At this point, the flap is replaced over the stromal bed. An OCT scan is performed 15 minutes later to confirm the position of the inlay. The implant is mostly invisible at the slit lamp at this stage, so OCT is required to confirm its position.
Surgeons familiar with LASIK and handling corneal flaps will find the TransForm procedure easy to learn.
CONCLUSION
My patients in the clinical trial and I were both extremely pleased with the results of this procedure. Based on my experience, I anticipate a new era in the corneal management of presbyopia.