CASE PRESENTATION
A 54-year-old woman presents approximately 6 weeks after blunt trauma to her left eye. The patient states that she was attacked in a bar and struck in her left eye with an unknown object. She noticed an immediate reduction in vision in the injured eye and was taken directly to the emergency department by ambulance. At the hospital, she was diagnosed with a ruptured globe and taken to the OR for treatment of a primary rupture. She was monitored closely by both anterior segment and retina specialists after the repair but recovered little vision.
Upon presentation, BCVA is 20/20 in the patient’s right eye and barely hand motions in her left. An afferent pupillary defect (APD) and irregular pupil are evident in the left eye. Slit-lamp examination of the right eye is normal. In the left eye, there is moderate conjunctival injection and a nasal limbal wound closed by multiple nylon sutures, several of which have exposed ends. The cornea is clear, and the anterior chamber has rare cells. The iris is irregular and peaked from tissue incarcerated in the nasal limbal laceration. A traumatic cataract is visible with irregular striations in the anterior lens capsule. No phacodonesis is evident, but mild hemorrhage is present on the lens capsule (Figures 1–4).
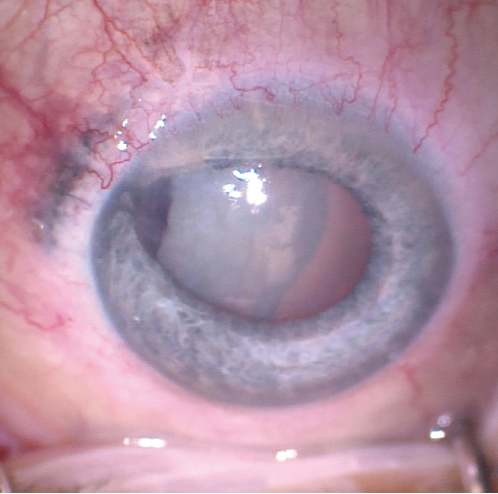
Figure 1. Nasal limbal laceration with iris incarceration and an irregular pupil in the left eye. The lens was cataractous with striations in the lens capsule and organized hemorrhage at the nasal aspect of the capsule.
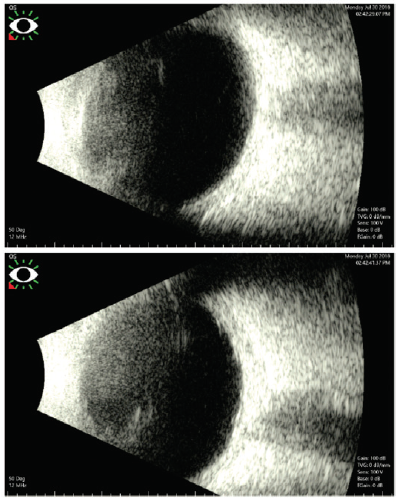
Figure 2. B-scan ultrasound images of the patient’s left eye.
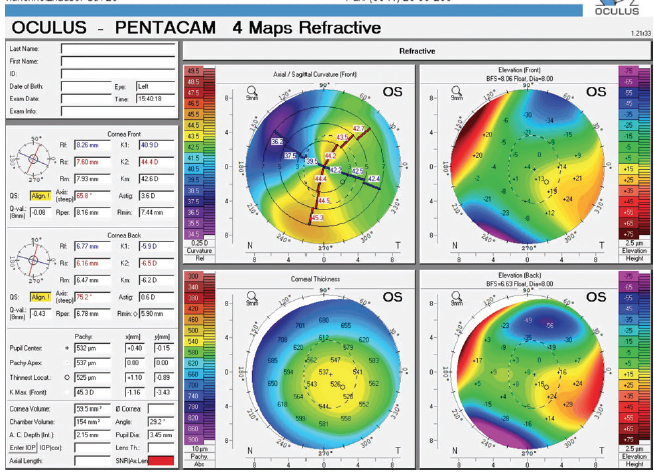
Figure 3. Tomography of the left eye showed flattening of the nasal cornea secondary to traumatic laceration.
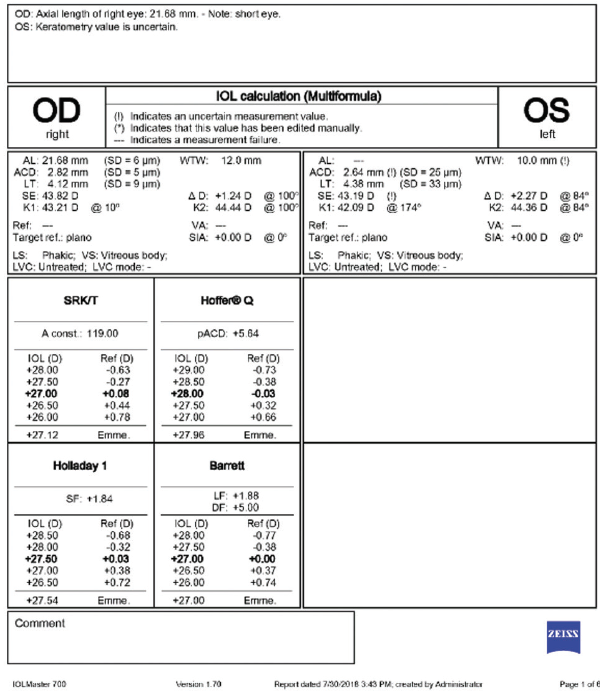
Figure 4. Biometry of the right and left eyes. Optical biometry could not accurately measure the axial length of the left eye. A-scan ultrasound showed an axial length of 21.7 mm in the left eye.
The patient asks if surgery can improve her vision. What factors must be taken into consideration in this case, and how would you proceed?
—Case prepared by Brandon D. Ayres, MD

NATALIE CHEUNG, MD, MS
The first step is to determine if surgery will improve this patient’s vision. B-scan ultrasound did not show any vitreous opacity, masses, or retinal detachment that would preclude her from seeing well. An APD was detected, likely indicating traumatic damage to either the optic nerve or retina. Other tools to determine visual potential include a red saturation test, laser interferometry, and potential acuity meter testing. Given the severity of the cataract, vision will likely improve but will be limited; I would not expect it to return to the pretrauma baseline. Additionally, significant corneal astigmatism may require the patient to wear glasses or contact lenses to achieve her best possible visual acuity. Because she may be anisometropic after surgery, informed consent should include discussion of this possibility and the need for postoperative management of the condition.
A-scan ultrasound confirmed a short axial length. Tomography showed nasal flattening in the area of limbal laceration and significant corneal astigmatism that was different from that shown by optical biometry. For this reason and because of the increased risk of zonular instability, I would not select a toric IOL. Instead, I would plan to implant a one-piece monofocal IOL in the capsular bag, and I would have on hand as a backup a three-piece monofocal IOL that could be placed in the sulcus in the event that capsular rupture or mild zonular dehiscence became evident. An iris-fixated IOL would not be an appropriate option in this case because of the preexisting incarceration of the iris. If a scleral-fixated IOL were needed owing to complete capsular instability or loss during surgery, I would consider staging the procedure.
Because I expect the case to be complex, I would plan on a retrobulbar block with monitored anesthesia care. I would create a temporal clear corneal incision, stain the capsule with trypan blue dye, and place iris hooks rather than a Malyugin Ring (MicroSurgical Technology) because pupillary dilation will likely be poor and/or irregular. I would not reopen and resuture the nasal laceration if it is well sealed.
Zonular instability could make performing the capsulorhexis difficult, so, if it were present, I would make a small capsulotomy. In the event of zonular dehiscence, I would consider placing capsular hooks after completing a continuous curvilinear capsulorhexis (CCC), and I would insert a capsular tension ring (CTR) before or after cortical cleanup, depending on the situation. Other techniques to minimize zonular stress include thorough hydrodissection to facilitate cortical cleanup and horizontal or vertical chopping for nuclear disassembly.
If the capsule is centered and intact, I would place a one-piece monofocal IOL in the capsular bag. Next, I would instill triamcinolone acetonide (Triesence, Alcon) to ensure that no vitreous had prolapsed into the anterior chamber. After the IOL was in place, I would consider gently tugging on the incarcerated iris with intraocular forceps to see if I could free up the tissue. Alternatively, I would leave the iris in place and consider performing a nasal pupilloplasty using either a modified Siepser sliding knot or the McCannel suturing technique.

DEAN OUANO, MD
An APD, a scleral laceration, and retropupillary hemorrhage after a blunt force injury to the globe are clinically significant signs of either the presence or future risk of a retinal detachment. As much as anterior segment surgeons like to operate autonomously, working closely with a vitreoretinal surgical colleague would be in this patient’s best interest. If the presence of a retained intraocular foreign body has not been ruled out, a helical or spiral computed tomography scan of the orbits with 3-mm axial and coronal cuts should be performed to rule it out.
There is an approximately 5-mm scleral laceration in the nasal quadrant, roughly parallel and 2 mm posterior to the limbus. The anatomic location of the scleral rupture likely corresponds to the iris root (or ciliary body, considering the eye’s relatively short axial length). Because uveal exposure to the conjunctival circulation is a known risk factor for the development of sympathetic ophthalmia, this scleral wound must be revised. Uveal tissue incarcerated in the incision should be removed. Gentle ab interno pulling of the displaced pupillary margin with Snyder forceps (MicroSurgical Technology) may normalize the iris architecture, after which the wound can be properly sutured with 10-0 nylon with the knots buried.
After performing a three-port pars plana vitrectomy (PPV) and lensectomy with appropriate management of posterior segment injuries, I would implant a posterior chamber IOL (CZ70BD, Alcon) and secure it with PTFE sutures (CV-8 Gore-Tex, W.L. Gore & Associates; off-label use) under superior and inferior Hoffman reverse scleral tunnels.

WHAT I DID: BRANDON D. AYRES, MD
The patient and I discussed at length the risks and benefits of cataract surgery and the increased complexity of the case, given her recent history of trauma. I explained the potential need for a PPV in the event of traumatically induced capsular or zonular damage. I also emphasized that her visual potential was uncertain because the presence of an APD indicated damage to the optic nerve, but I stated that the extent of the damage could not be estimated. The patient understood the increased complexity and risk of surgery but was highly motivated to improve her vision and agreed to surgery.
My plan was to remove the cataract, potentially requiring PPV, and to repair the iris, if possible. To improve visualization of the anterior capsule, I instilled trypan blue dye into the anterior chamber and then rinsed it with balanced saline solution (Figure 5). It subsequently became clear that the anterior capsule was intact, so I created a small CCC with a cystotome and Utrata forceps (Figure 6).
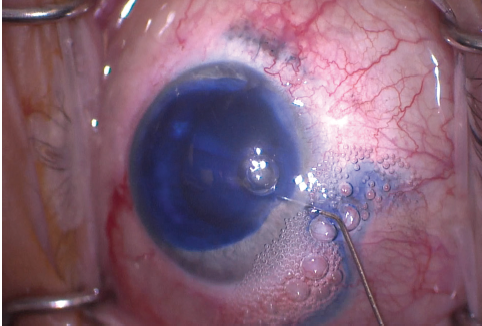
Figure 5. The anterior capsule was stained with trypan blue dye to improve visualization.
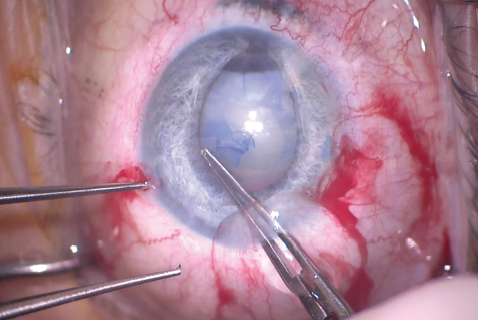
Figure 6. The surgeon used Utrata forceps to make the capsulorhexis. The irregular pupil made creation of a large capsulorhexis difficult.
Next, I used bimanual I/A to aspirate the lens material; because of the softness of the lens, no phacoemulsification was necessary. The red reflex then allowed improved visualization of the capsular bag. No capsular defect was evident—a pleasant surprise—which meant that an IOL could be placed in the capsular bag. No vitreous prolapse or severe zonulopathy was encountered during the lens removal portion of the surgery.
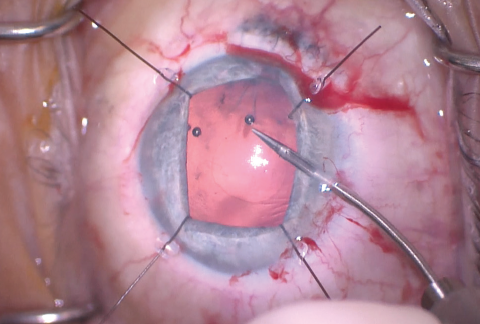
Figure 7. Flexible iris retractors were used to improve access to the anterior capsule, and microscissors were used to cut the edge of the capsulorhexis, allowing enlargement of the CCC.
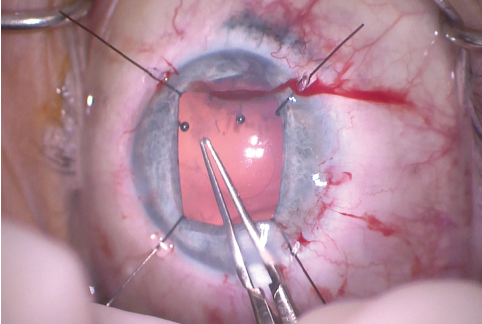
Figure 8. The Utrata forceps were used to enlarge the capsulorhexis.
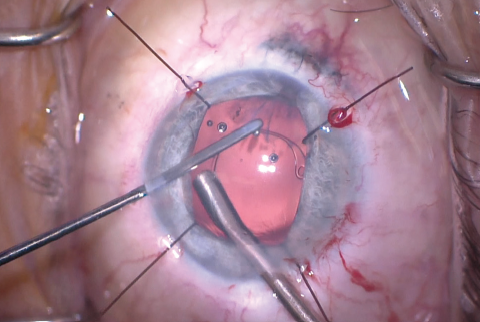
Figure 9. A CTR was inserted after enlargement of the CCC.
The size of the original CCC had been limited by pupillary size, so the capsular opening was enlarged in order to prevent capsular phimosis. To improve visualization, I placed flexible iris retractors and then used microscissors and Utrata forceps to enlarge the CCC (Figures 7 and 8). After placing a CTR in the capsular bag, I implanted the IOL (Figures 9 and 10). With the implant securely located within the capsular bag, I removed the flexible iris retractors, allowing the iris to recover its original configuration. I administered a miotic agent to constrict the pupil. With microforceps, I tested the elasticity of the iris tissue to ensure that an iridoplasty could be performed without putting too much tension on the iris (Figure 11). I found that the iris had retained significant elasticity, and I placed a 10-0 polypropylene suture to create a more centered pupil (Figure 12).
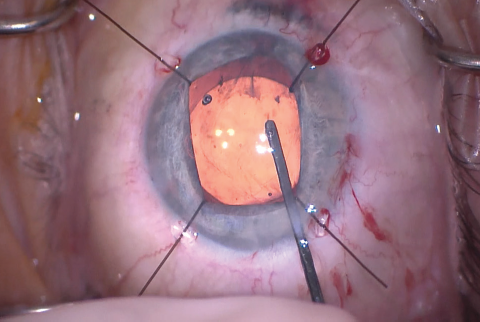
Figure 10. The IOL was inserted into the capsular bag. The stability of the capsular bag allowed excellent centration.
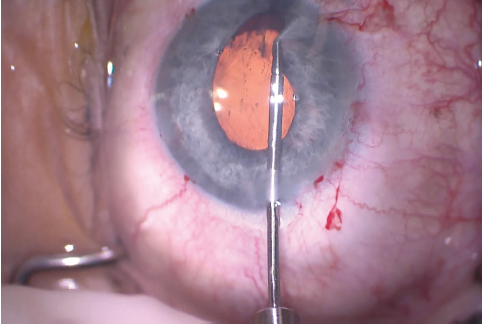
Figure 11. The surgeon used microforceps to check the elasticity of the iris tissue and to make sure an iridoplasty could be performed. Microforceps can also be used to show where a polypropylene suture should be placed to minimize the irregularity of the pupil.
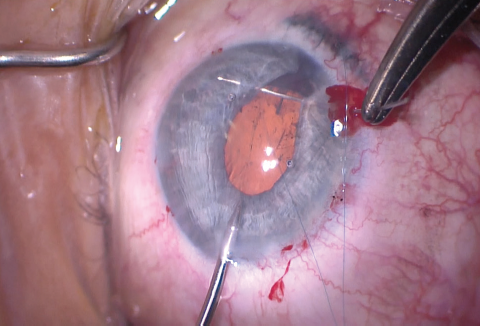
Figure 12. A 10-0 polypropylene suture on a curved needle was used to make an iridoplasty to re-create the pupillary border.
Placement of a single iris suture often leaves the pupil looking quite peaked. The irregular shape may be cosmetically insignificant when the color of the tissue is dark, but a misshapen pupil is highly noticeable in an eye with a blue iris. To help shape and center the pupil, I used intraocular diathermy on a very low setting (10%–15% of maximum bipolar coagulation) to cauterize and sculpt the pupil. The thermal constriction of the iris fibers rounded out and centered the iridoplasty (Figure 13). I removed the OVD from the anterior chamber with the I/A unit and ensured that the incisions were watertight.
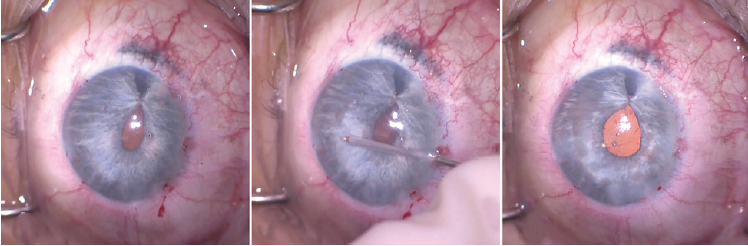
Figure 13. The shape of the pupil after placement of an iridoplasty suture. Note the peaking of the pupil at the site of the suture (left). Intraocular diathermy was used to shape the pupil (center). Pupillary shape is shown immediately after use of cautery to round out the pupillary margin. Small transillumination defects were visible in the areas of the iris where cautery was used (right).
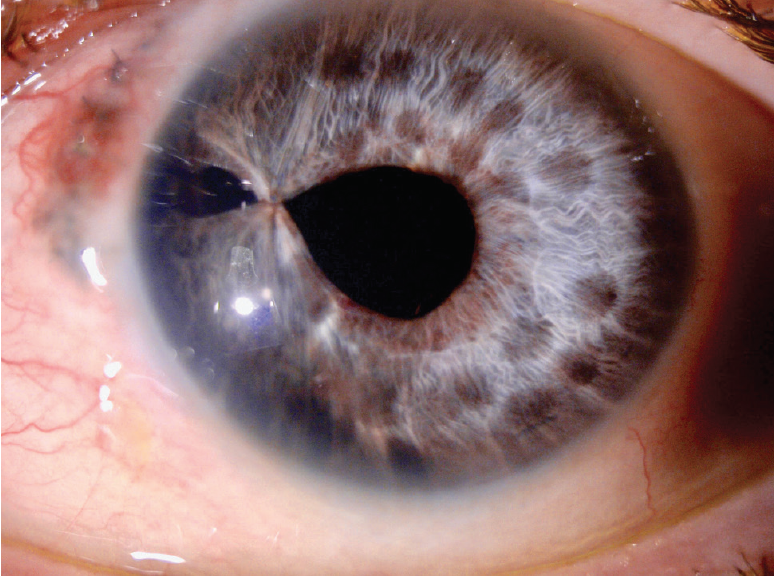
Figure 14. Three months after surgery, a slit-lamp examination of the left eye showed the iridoplasty suture and several round areas of depigmentation from iris cautery. Note the stability of the thermal damage from immediately after surgery (Figure 13) to 3 months postoperatively. Several nylon sutures from the patient’s previous ruptured globe repair were visible at the nasal limbus.
The patient tolerated the surgery very well, and the postoperative course was uneventful. One day after surgery, UCVA in the left eye had returned to 20/40, and it has remained stable over the past 3 months (Figure 14).




