Advances in technology should incentivize surgeons to consider novel surgical techniques. For me, the availability of the femtosecond laser for cataract surgery has contributed in this way, leading to changes in my surgical approach to nucleus removal.
Several aspects of femtosecond laser technology have allowed me to travel this new path:
- The imaging of the lens provided by these devices allows the laser to cut deep into the lens while avoiding violation of the posterior capsule;
- The laser’s precision leads to consistent size and shape of the capsulotomy and specific patterns of fragmentation in the lens; and
- The reproducibility of the laser and imaging sets the stage for the surgeon so that, case after case, the strategy for lens removal is more predictable and consistent and the surgeon’s comfort level increases.
Laser cataract surgery technology may ease the surgeon’s anxiety in cases in which there is heightened concern about the integrity of the capsular bag, such as in patients with a history of trauma or previous surgery, pseudoexfoliation, or specific medical conditions. In cases such as these, zonular dehiscence can complicate surgery and require a vitrectomy, sewn IOL, or capsular tension ring. The laser lessens some of these worries by allowing us to reduce stress on the capsular-zonular complex.
LOOK BUT DON’T TOUCH
There are many aspects of nucleus removal in traditional cataract surgery that can put stress on the capsular bag and contribute to progressive weakening of the zonules. Traditional surgery requires manual pulling on the anterior capsule to perform the capsulorhexis. The vector force applied while ripping will indirectly pull on the zonules in the opposite direction.
Additionally, the size and shape of the rhexis are not always consistent, especially when the capsular bag is loose. If a larger rhexis is created, it leaves less shelf for sulcus IOL placement if one becomes necessary later in the case. A smaller rhexis, on the other hand, will likely create more force pulling on the bag as pieces of the lens nucleus are moved into the anterior chamber, maybe even causing a rent in the anterior capsule.
Techniques in which sculpting is used to crack the lens create additional force on the bag as the surgeon pushes the phaco needle into the lens to create a groove. Additionally, the cracking of the lens itself pushes the nuclear pieces firmly against the walls of the bag. Once the quadrants are created, they are large, and pulling each one into the anterior chamber adds to the pulling force on the capsular bag as the piece is fit through the rhexis opening.
Even the pop-and-chop technique requires pulling the entire nucleus through an opening much smaller than the lens diameter and, again, this may significantly pull on the bag. These techniques typical of traditional cataract surgery contribute to overall tension and manipulation of the capsular bag.
EVOLVING TECHNIQUE
Initially, I used the femtosecond laser as an adjunct to my traditional nucleus removal strategy. However, over time, the technology has allowed me to evolve to a technique I would never have considered in a traditional case. Surprisingly, it is the integrated imaging that is, in my opinion, more powerful than the actual laser. The imaging allows me to use the laser cuts to aid in nucleus removal with less manipulation and tension on the capsular bag. Following are the reasons supporting this claim, which can also be seen in the video below.
First, rhexis creation is improved. The capsular button is free 97% of the time, which results in no pulling on the capsular bag (Figure 1). The rhexis is round and of consistent size in each case. My manual capsulorhexis is close, but it is often slightly oval or heart-shaped.
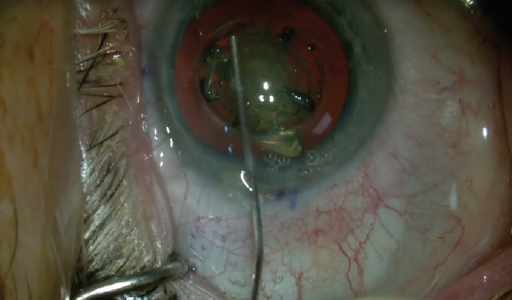
Figure 1. Capsulorhexis: A canula is used to press on the cut capsular button to confirm it is free and then placed under the button to scoop it up into the anterior chamber. One must also verify that tags are not present.
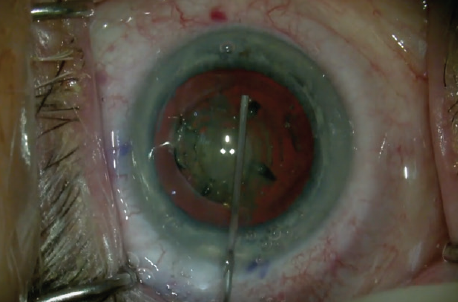
Figure 2. Viscodissection: Viscoat is injected 270º under the anterior capsule only to the equator. The laser-created air bubble pneumodissects posteriorly.
Second, one can avoid hydrodissection and can use viscodissection to the equator. This is done slowly, deliberately, and with great control around approximately 270° to completely free up the anterior capsule, while the posterior capsule is pneumodissected by the air bubble created by the laser (Figure 2). The placement of Viscoat OVD (Alcon) also creates a space for a second instrument to move and perform cracking. In manual cataract surgery with hydrodissection, there is an impressive fluid wave that frees up posterior lens adhesions; however, it only minimally addresses anterior adhesions.
Third, the laser can cut the core of the nucleus in a specific manner, reducing the need to manipulate it during removal. With my settings, the laser cuts a cylinder with a diameter of 4.8 mm and a plus-sign chop pattern 5.3 mm in length, oriented to align with my primary corneal incision. Overall, a total of five cylinders are included in the pattern, and imaging permits the laser to cut deep into the lens, to within only 250 µm from the posterior capsule (Figure 3).
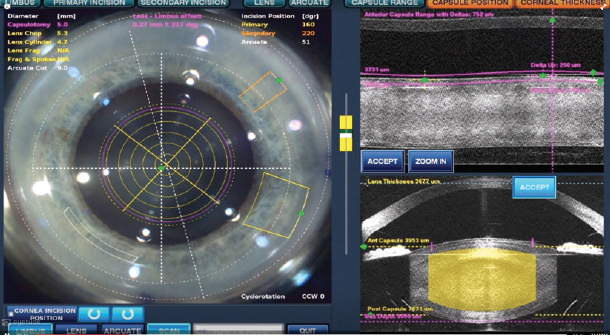
Figure 3. Laser cuts: The OCT image of the lens allows customization of laser cuts to be consistently within 250 μm of the posterior capsule.
Fourth, the technology then transfers from the laser room into the OR. I have incorporated new settings on my phaco system for cases in which I use the femtosecond laser. Specifically, these settings use high vacuum; no longitudinal phaco; and linear-controlled, pulsed torsional phacoemulsification if necessary.
The lens is not pushed or pulled during this part of surgery, as I bowl out and remove the denser core of the lens that has already been cut by the laser, using mostly vacuum (Figure 4). In contrast, grooving a lens that has not been precut by the laser requires lens movement and pressure on the capsular bag.
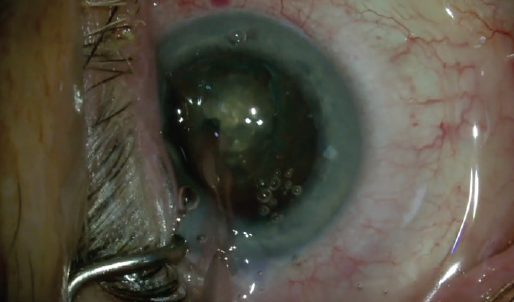
Figure 4. Bowl lens: Mostly aspiration and minimal phaco energy are typically necessary, with the laser cutting the dense nucleus into many pieces deep into the lens. The edges of the bowl are laser cut vertical and smooth to ease cracking.
Cracking of the nucleus is simplified with the laser cut pattern. When laser technology has been used in previous steps of the procedure, cracking is easier because the bowling is 4.8 mm in diameter and the chop pattern extends to 5.3 mm. Because the edge of the bowl is laser-cut and vertically flat, the second instrument can effectively move the quadrants for cracking (Figure 5).
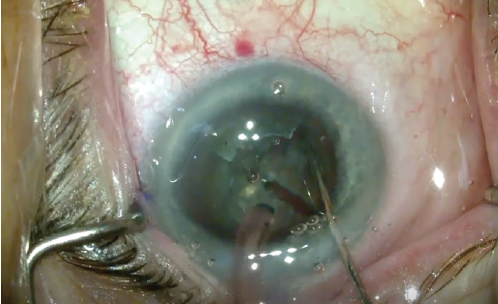
Figure 5. Creating quadrants: The bowled-out nucleus with minimal posterior lens remaining allows cracking of the lens with minimal stress on the bag.
The quadrants are created gently, with minimal manipulation into the bowled-out area, still in the bag, as their size at this point is only 2 to 3 mm. There is room around the quadrants for the second instrument, as created during the viscodissection step, and they have already been scored by the laser cut. The bowling of the lens, 4.8 mm wide and more than 90% of lens depth, leaves minimal epinuclear plate to restrict quadrant movement.
Quadrant removal is also simplified. In traditional surgery, sculpting is limited and cautious because of the fear of rupturing the posterior capsule. As a result, often a thicker posterior plate remains, adding to the limitations of moving the quadrants. Additionally, the quadrants must be pulled out of the bag and closer to the cornea for ultrasound removal because the lens is not bowled out.
In laser-treated lenses, by contrast, each quadrant can be pulled into the already bowled-out area, and they fit easily into this space still within the bag (Figure 6). Thus, the quadrant does not have to be pulled into the anterior chamber and can be removed within the bag. This reduced amount of movement in a laser-cut nucleus places minimal tension on the bag and implements what I call a no-touch technique for the capsular bag. This in turn places less stress on the capsular bag and potentially leads to fewer complications, especially in higher-risk patients.
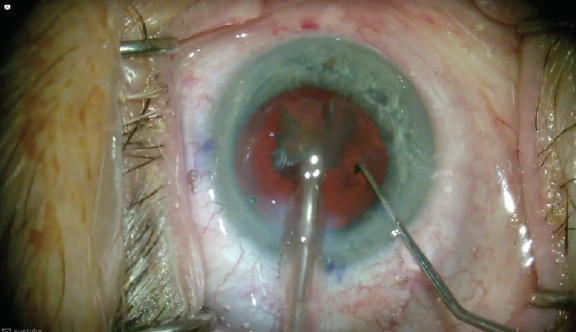
Figure 6. Ease of quadrant removal: The bowled-out area creates a workspace in the bag. The quadrants can easily be pulled central for removal. Note that the entire lens is never rotated in the bag and there is minimal tension on the zonules during lens removal.
CONCLUSION
Ophthalmologists who are performing laser cataract surgery should think about using these new tools to approach surgery differently. Taking advantage of the imaging available on these laser systems can be beneficial, and the laser cut patterns can facilitate incorporating novel techniques such as those described here. Challenge yourself to take advantage of the precision and accuracy these lasers offer to modify your current surgical technique.




