Presbyopia-correcting intraocular lenses (PCIOLs) can offer excellent visual outcomes at multiple distances and, in some patients, minimize the need for spectacles. Many of these PCIOLs have relied on diffractive optics to extend the range of vision, and were frequently associated with clinically significant increases in visual disturbances relative to monofocal IOLs. These PCIOL options are usually limited to patients without common comorbidities, such as prior refractive surgery, dry eye, or mild glaucoma, due to the associated visual disturbances.1 The AcrySof IQ Vivity® IOL (Alcon) is an extended depth-of-focus (EDOF) IOL with a unique wavefront shaping technology, which provides an extended range of vision while maintaining low incidence of visual disturbances.2-4 The AcrySof IQ Vivity® IOL offers an extended depth of focus with wavefront-shaping technology, which stretches and shifts light rather than splitting it.2,3
There are two surface transition elements in the center of the AcrySof IQ Vivity® IOL that modify the wavefront coming through the lens. First, a slightly elevated smooth plateau (~1 μm) stretches the wavefront resulting in a continuous extended focal range. Second, a small change in curvature shifts the wavefront so that all light energy is used.2,3 The beauty of this IOL is that it was designed to eliminate unwanted optical phenomena, such as glare and halos, that come from the splitting of light in a diffractive IOL, and which tend to be particularly aggravating in patients with many common comorbidities to cataracts.2,3
Two protocol-driven studies (one performed in the United States and the other outside of the United States) clearly showed that the AcrySof IQ Vivity® IOL has better uncorrected visual acuity for intermediate and near distances, and a similar visual acuity as the AcrySof IQ monofocal for distance focus, while maintaining low incidence of visual disturbances.2,3 Therefore, we know that the AcrySof IQ Vivity® IOL works in healthy eyes without other conditions. Now, because of the excellent and consistent data from the AcrySof IQ Vivity® IOL Registration Studies, we see that the AcrySof IQ Vivity® IOL can be used successfully in a much broader range of subjects.
Real-World Data Collection
Miguel Teus, MD: A registry study is typically an observational, non-interventional study collecting data from a given case area. The surgeon decides what treatment to apply, what kind of IOL to implant, and what data are collected. Registry studies are different from interventional studies, in which a specific subset of subjects is all treated according to the same strict protocol. While interventional studies are necessary to tease out the impact of a specific treatment protocol, registry studies often better reflect the real world, where subjects with complications and comorbidities still need treatment. The AcrySof IQ Vivity® Registry Study* provided valuable data across a wide section of cataract patients in a real-world setting. At the recent ESCRS meeting in Milan, Italy, Miguel Teus, MD; Ramin Khoramnia, MD; and Ruth Lapid, MD, reviewed the results of the second interim analysis of the AcrySof IQ Vivity® IOL Registry Study.
AcrySof IQ Vivity® IOL Registry Study Overview
Ramin Khoramnia, MD: Everyone on this panel participated in the multicenter, ambispective, non-comparative, open-label, non-interventional AcrySof IQ Vivity® IOL Registry Study. The study is being conducted across 43 sites from 8 countries: Australia, Belgium, Germany, New Zealand, the Netherlands, Portugal, Spain, and the United Kingdom. Any subject could enter the trial, provided the AcrySof IQ Vivity® IOL was implanted in both of their eyes. There were two main exclusion criteria: subjects could not be pregnant and they could not have had corneal refractive surgery after lens implantation.
The primary endpoint was photopic binocular uncorrected visual acuity at distance, and various exploratory effectiveness endpoints were also collected. These included photopic binocular uncorrected visual acuity at intermediate and near distances, as well as photopic binocular corrected visual acuity at distance, intermediate, and near. We also looked at the residual refractive error, assessed subject satisfaction and spectacle independence, and assessed visual disturbances by asking open, non-prompted questions about visual experience.
More than 650 cataract subjects were implanted bilaterally with AcrySof IQ Vivity® and/or AcrySof IQ Vivity® Toric IOLs. In this study, subjects had a mean uncorrected distance visual acuity of 0.02 logMAR, or approximately 20/20 Snellen. The lens performed extremely well at intermediate distances with a visual acuity of 0.088 logMAR, or 20/25 Snellen, and the near-distance performance was reasonable at 0.253 logMAR, or 20/32-3 Snellen.
Subject satisfaction and spectacle independence were also very high (Figure 1). Subjects never or rarely needed glasses in bright and dim light conditions, and most did not require glasses to see far away or at arm’s length in either bright or dim light conditions. Approximately 60% of subjects never or rarely required glasses for near-distance activities.
One of the most interesting findings of the Vivity® Registry Study demonstrates that the AcrySof IQ Vivity® IOL has minimal photic phenomena in this real-world assessment study. More than 91% of subjects reported no halos, no glare, and no starbursts (Figure 2). These results are promising because PCIOLs are often associated with such side effects. Dr. Lapid, did you have a similar experience?
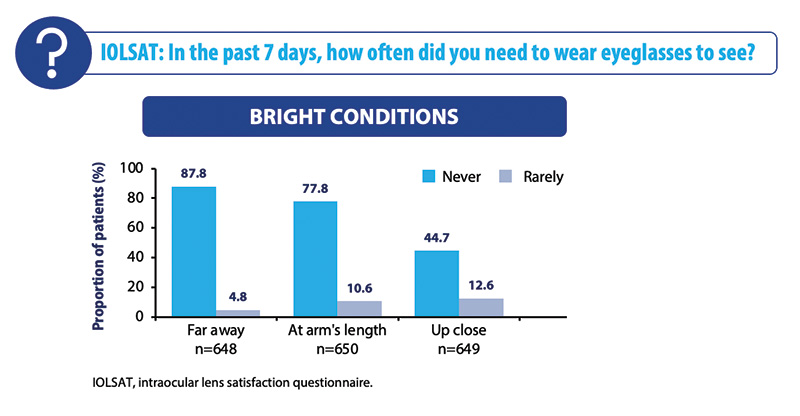
Figure 1. Spectacle independence at 3 to 6 months under bright conditions in the overall cohort.
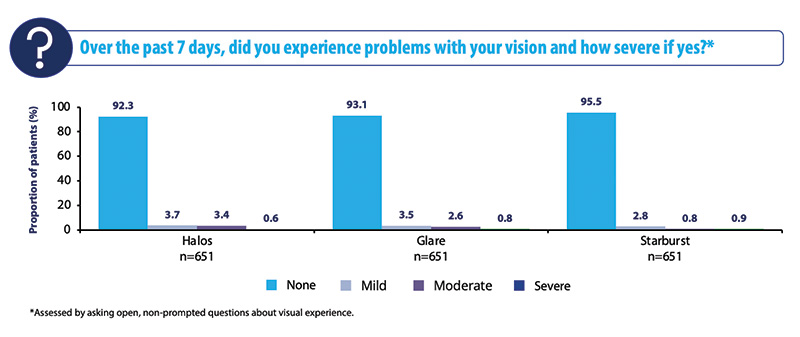
Figure 2. Visual disturbances in the overall cohort at 3 to 6 months.
Ruth Lapid, MD, PhD: My own cohort mirrors the overall registry study data. Historically, I used to implant monofocal IOLs in about 6-8% of patients, because I believed some patients would not tolerate the visual disturbance profile of multifocal IOLs, and we had quite a few patients that we had to disappoint. The AcrySof Vivity® IQ IOL has allowed me to broaden presbyopia management options to these patients. This includes appropriate subjects with irregular corneas and the post-laser subjects whom we were unsure about. I have been implanting a number of these patients with the AcrySof IQ Vivity® IOL with excellent results as demonstrated in the data reported as part of the Vivity® Registry. As a surgeon I’m very satisfied, and the availability of new technology such as the AcrySof IQ Vivity® IOL is allowing me to consider patients that I’ve previously excluded from PCIOL consideration.
Prof. Khoramnia: We had a similar experience. In the beginning, I thought that this lens would only be considered for patients who otherwise would have received a diffractive IOL. I still think if a subject is a suitable candidate for a full range of vision diffractive IOL, like the PanOptix IOL (Alcon), we should still recommend the trifocal lens. However, the AcrySof IQ Vivity® IOL is certainly a lens to consider for subjects to whom we would have said, ‘You probably are not a good candidate to undergo surgery with the PCIOLs,’ either because they have relevant comorbidities or they are not willing to accept the side effects.
Monovision Cohort
Prof. Teus: This IOL has quite a flat defocus curve around plano. Dr. Lapid, do you think this has some benefit considering mini-monovision?
Dr. Lapid: The flat defocus curve around plano gives me confidence on achieving successful mini-monovision since I can target the non-dominant eye slightly myopic without sacrificing distance vision performance significantly. Using a slight offset further extends the depth of focus and allows patients to achieve spectacle independence in more real-world situations. In fact, I do mini-monovision in the majority of my Vivity® patients.
In the AcrySof IQ Vivity® Registry Study, about 20% (135 subjects) of the subjects met the criteria for monovision, which was defined as at least one eye with manifest refraction spherical equivalent of less than or equal to -0.5 D and an absolute difference in mean manifest refraction spherical equivalent between eyes greater than or equal to 0.5 D. In terms of uncorrected binocular photopic visual acuity for far and intermediate distances, subjects reached a visual acuity of logMAR 0.04, which is about 20/20 minus two letters. Near vision was 0.219 logMAR, which is about 20/32. This is a wide range of functional vision.
In the Vivity® Registry Study, if we compare the uncorrected visual acuities for the mini-monovision cohort and without mini-monovision, the mini-monovision cohort may lose two letters for the uncorrected visual acuity for distance, but they may gain intermediate and functional near vision (Figure 3). This is quite extraordinary because these subjects were largely free of glasses for distance and intermediate, and surprisingly, 60% of our patients achieved functional near without glasses (Figure 4). Satisfaction rates mirror that of the overall cohort with over 91% satisfied with their current vision and more than 91% reporting no photopic phenomena.
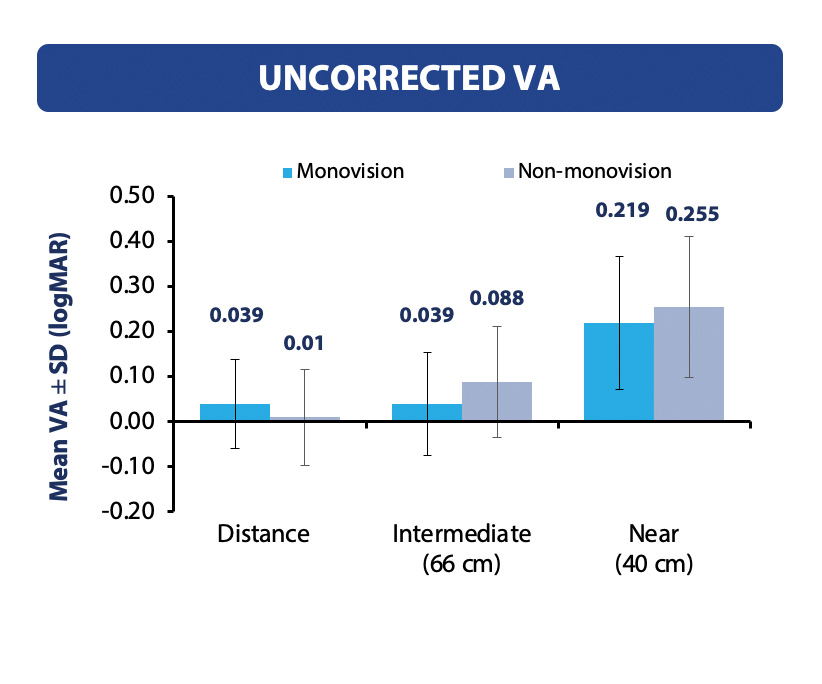
Figure 3. Binocular uncorrected visual acuity at 3 to 6 months mini-monovision vs non-monovision cohort.
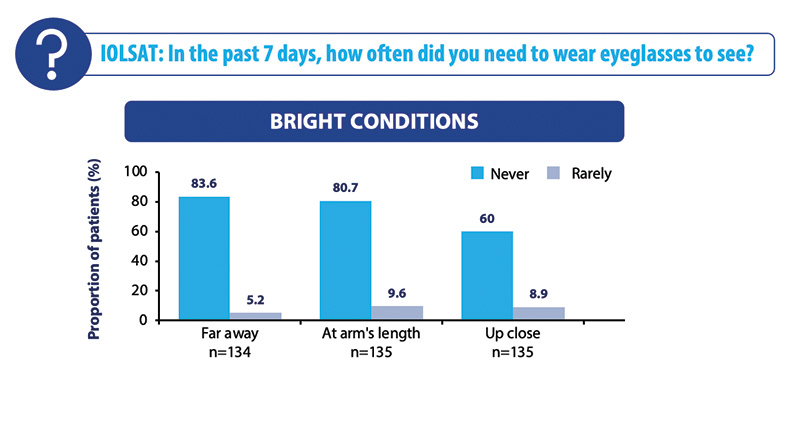
Figure 4. Spectacle independence under bright light conditions in the mini-monovision cohort.
Post-Myopic corneal refractive surgery Subjects
Prof. Teus: We know that patients who had previous refractive surgery for myopia often do not have outcomes as good as people who never had laser surgery, particularly with diffractive PCIOLs.5 Dr. Lapid, what is your experience with the AcrySof IQ Vivity® IOL in this group of patients?
Dr. Lapid: The refractive outcome can be less predictable in post-laser eyes with more difficult, oblate, or hyperprolate corneas. I find that the defocus curve of the AcrySof IQ Vivity® IOL, specifically the relative flatness around plano, gives me greater confidence of achieving good distance vision. The AcrySof IQ Vivity® Registry included a cohort of post-myopic laser subjects (n = 20). The mean uncorrected visual acuity was 0.1 logMAR, or 20/25 Snellen, which is still functional distance vision. These subjects also achieved intermediate visual acuity of 0.079 logMAR, or 20/25 Snellen. Near visual acuity was 0.208 logMAR, or 20/32 Snellen, so subjects were able to achieve functional near vision (Table 1).
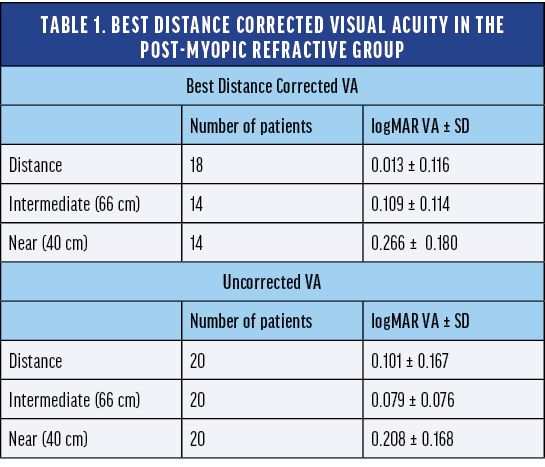
Post-myopic subject satisfaction following AcrySof IQ Vivity® IOL implantation was 85% in the Vivity® Registry Study. This is a high percentage; it is just slightly below the satisfaction rate of the overall cohort in the Vivity® Registry Study.
Like the overall cohort, more than 90% of patients in the post-myopic cohort reported no visual side effects (Figure 5). This is another encouraging statistic because subjects who previously had laser surgery tend to be more demanding than subjects who have not.
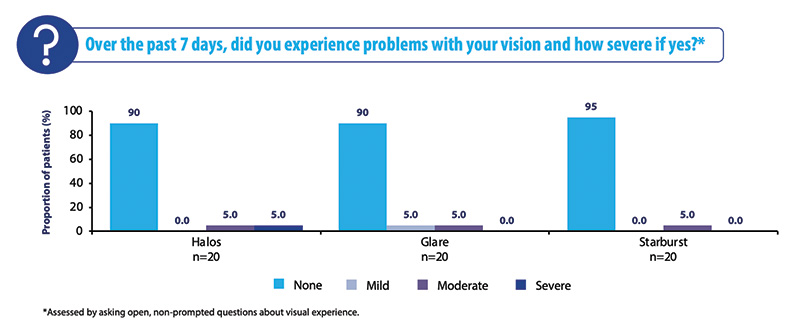
Figure 5. Visual disturbances at 3 to 6 months in the post-myopic cohort.
Dry Eye Subjects
Prof. Khoramnia: We routinely use presbyopia-correcting lenses in healthy eyes, but in the real world, we have a lot of subjects who do not have healthy eyes. The Vivity® Registry Study analyzed subgroups of subjects with dry eye and early glaucoma and is helping us to offer PCIOLs to patients with comorbidities that we may have previously just excluded.
Prof. Teus: Preoperative dry eye is the most common comorbidity and is traditionally considered a contraindication for PCIOLs, as an unstable optical surface may increase visual disturbances (e.g., glare, halo, starburst).6 Dry eye has also been reported to be highly correlated to post-cataract patient dissatisfaction.7
The Vivity® Registry Study dry eye cohort included 62 subjects. The uncorrected visual acuity outcomes (distance, 0.03 logMAR; intermediate, 0.086 logMAR; near, 0.218 logMAR) were similar to the overall cohort. Approximately 50% of subjects did not need glasses for near activities, and the vast majority did not need glasses for far or intermediate vision. Overall satisfaction was also good in subjects with preoperative dry eye disease, with 79% (49/62) of patients with dry eye reporting satisfaction with their sight at present.
The most interesting and surprising result in this cohort was that unwanted visual phenomena were comparable to the overall cohort, with over 90% of subjects reporting no halos, glare, or starburst (Figure 6). Visual disturbances are the main complaint for dry eye subjects with a multifocal IOL or even with a monofocal IOL. While these results are very encouraging, surgeons should continue to use their own clinical judgement for individual patients, as more research is needed in this area.
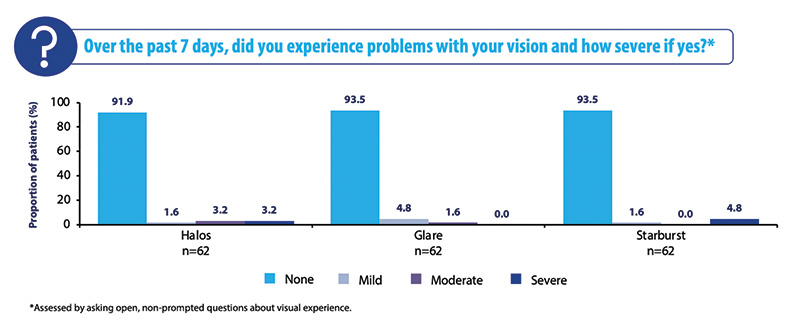
Figure 6. Visual disturbances at 3 to 6 months in the dry eye cohort.
Glaucoma Subjects
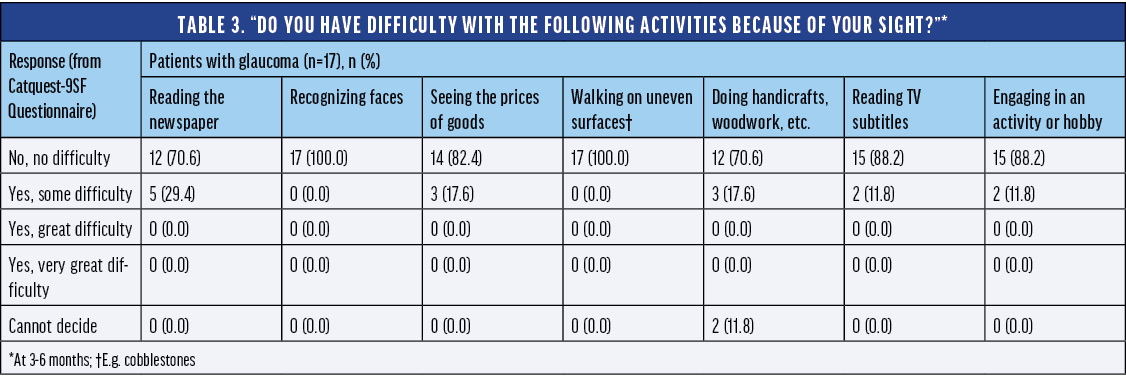
Prof. Teus:Subjects with mild glaucoma also demonstrated very good results using the AcrySof IQ Vivity® IOL. The registry study included 17 subjects with mild to moderate glaucoma. The uncorrected and best distance-corrected visual acuity for distance, intermediate, and near vision were comparable to the overall cohort (Table 2).
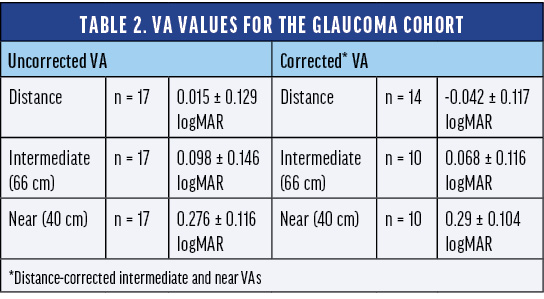
The glaucoma cohort performed quite well in terms of spectacle independence and subject satisfaction. More than 94% of subjects with glaucoma did not need to wear eyeglasses to see far away or at arm’s length in bright or dim conditions, and 65% never or rarely needed eyeglasses for up-close tasks in bright conditions after implantation of the AcrySof IQ Vivity® IOL. An impressive 100% of subjects with glaucoma were very satisfied with their sight at present (Table 3). None of the glaucoma subjects reported starburst, and most reported no halos or glare. Once again, it is really encouraging to see these types of results as we continue to research the impact of these technologies in specific patient populations.
Patient Counseling
Prof. Khoramnia: With cataract and refractive surgery, it is wise to under-promise and over-deliver. I advise my Vivity® patients that they may need spectacles, especially for near activities. The study showed a good rate of subjects who did not require glasses for near visual tasks, but 40% of subjects did need glasses for some near vision tasks.
The Vivity® Registry Study showed excellent results for photic phenomena; however, I still recommend counseling patients with any type of IOL, including monofocals, that photopic phenomena can occur. Even though 90% or more do not perceive it, that means there are 10% who do. Though a minority, these patients could be unhappy if they are promised prior to surgery that they would not experience photic phenomena. We need to keep in mind that there are some patients who suffer from photic phenomena even with a monofocal lens. I always tell my patients opting for the AcrySof IQ Vivity® IOL that we see minimal reports of bothersome photic phenomena; however, they are likely to get better visual acuity outcomes at intermediate and near distances with the AcrySof IQ Vivity® IOL. Prof. Teus, you spoke earlier about the outcomes for patients with dry eye and glaucoma comorbidities. What do you say to your patients in these cohorts when you are considering implanting the AcrySof IQ Vivity® IOL?
Prof. Teus: The Vivity® Registry Study was a real-world study, so there was not a strict definition of dry eye. We do not know if the subjects had mild, moderate, or severe dry eye. When I see a patient with moderate to severe dry eye, I tell them that they are not suitable for a diffractive IOL, but that the AcrySof IQ Vivity® IOL may provide them with more visual benefits than a monofocal, particularly if their ocular surface disease is managed. Regardless of the severity, it is always recommended to address dry eye before surgery to help set patients up for success, no matter the lens type.
I see a lot of glaucoma patients in my practice. If the patient has mild visual field defect and good central visual field function without any defect close to fixation, I still consider them candidates for the AcrySof IQ Vivity® IOL, and they can do very well with the implant.
Ease of Use
Prof. Teus: Other types of PCIOLs that work using diffractive optics or a high level of negative spherical aberration or similar phenomenon require us to carefully analyze the eye preoperatively, and have rigorous preop discussions related to visual dysphotopsias. Do you think that the AcrySof IQ Vivity® IOL is easier to use and discuss with patients?
Prof. Khoramnia: I agree that the AcrySof IQ Vivity® IOL is an easy lens to use and discuss with patients. I frequently implant diffractive IOLs, and implanting this type of lens presents some additional considerations compared to a monofocal IOL. We must dedicate a significant amount of time to educating our patients about the photic phenomena that they can experience after surgery, and it is difficult to simulate what their vision will be like after surgery. However, by selecting the right patients and managing their expectations through education, they are happy even if they do experience photic phenomena. Unfortunately, not all surgeons can spend as much time counseling. The AcrySof IQ Vivity® IOL can be an important solution in these situations because a surgeon can now offer patients the benefits of an increased range of vision with less time spent counseling patients compared to other PCIOL types.
I think that one of the reasons for the success of the AcrySof IQ Vivity® IOL is that we do not have to spend that much time on pre- and postoperative patient counseling. I am very happy using trifocal IOLs, but I do have to thoroughly examine the eyes and discuss with the patients their willingness to accept side effects to determine whether patients are suitable candidates. If I do not, I will have unhappy patients.
Dr. Lapid: I agree that patient counseling is very important, and that the AcrySof IQ Vivity® IOL is making life a little easier for us.
Patient Choice
Prof. Teus: In my opinion, every cataract patient should hear that they have at least three possibilities—a monofocal, a PCIOL, and monovision. Then they can choose from these possibilities. What are your thoughts?
Dr. Khoramnia: I completely agree that we should always inform our patients about the lens options that we have. When I have a patient who is not suitable for a trifocal lens, I tell them, ‘There is a trifocal lens on the market, but for your eye, this is not an option.’ However, the AcrySof IQ Vivity® IOL is changing this conversation. Many patients who are not good candidates for a trifocal IOL are now good candidates for the AcrySof IQ Vivity® IOL.
Prof. Teus: On the other hand, patients who want to completely get rid of glasses must accept some possibility of having photic phenomena as a trade-off for the chance of not requiring glasses for most near activities. If a patient is not willing to accept the risk profile in terms of photic phenomena, then the AcrySof IQ Vivity® IOL is indicated over a diffractive lens.
Dr. Lapid: Since I added the AcrySof IQ Vivity® IOL, the number of monofocal IOLs I implant in the cataract-refractive clinic is declining. Do you see a place where the AcrySof IQ Vivity® IOL could make monofocal lenses obsolete?
Prof. Khoramnia: I only very rarely have patients who say, ‘I really love my glasses. Please allow me to have my hyperopia and presbyopia after surgery.’ The vast majority of patients would be thrilled to have extended depth of vision as the standard of care. However, there are still comorbidities that exclude even the wavefront shaping technology of the AcrySof IQ Vivity® IOL. The Vivity® Registry Study is generating data to support a broader group of patients than studied in the registration trials can be successful with the AcrySof IQ Vivity® IOL; however, I am a conservative surgeon, so I want to see more data before I am completely comfortable with stating that the AcrySof IQ Vivity® IOL can replace a monofocal option.
Prof. Teus: We do have a lot of data about this lens from both real-world and prospective trials, and the AcrySof IQ Vivity® Registry Study is an important source of information to understand how patients with comorbidities may perform with the AcrySof IQ Vivity® IOL. But like Dr. Lapid, in my practice the number of monofocal IOLs I implant is really going down.
The Vivity® Registry Study results to date are quite good across all cohorts studied, and the data show that the AcrySof IQ Vivity® IOL is an option for a broader range of patients.
Conclusion
Prof. Khoramnia: The Vivity® Registry Trial is a real-world clinical study involving more than 650 cataract subjects. The study has shown that the AcrySof IQ Vivity® IOL provides excellent distance and intermediate vision and functional near visual acuity results with high spectacle independence, subject satisfaction, and limited visual disturbances for the overall population. We also looked at mini-monovision, post-myopic corneal refractive, dry eye, and glaucoma subject subgroups and we saw comparable outcomes to the overall cohort.
The AcrySof IQ Vivity® IOL can be successfully implanted without the need for expensive, specialized equipment or extensive patient counseling. The AcrySof IQ Vivity® IOL has great potential for patients who otherwise would not be candidates for multifocal lenses and certainly expands the pool of patients who are able to receive presbyopia-correcting technology.
1. Kohnen T, Suryakumar R. Extended depth-of-focus technology in intraocular lenses. J Cataract Refract Surg. 2020;46:298–304.
2. Bala C, Poyales F, Guarro M, et al. Multicountry clinical outcomes of a new nondiffractive presbyopia-correcting IOL. J Cataract Refract Surg. 2022;48:136–143. doi: 10.1097/j.jcrs.0000000000000712.
3. McCabe C, Berdahl J, Reiser H, et al. Clinical outcomes in a U.S. registration study of a new EDOF intraocular lens with a nondiffractive design. J Cataract Refract Surg. 2022;48:1297–1304. doi: 10.1097/j.jcrs.0000000000000978.
4. AcrySof® IQ Vivity® IOL Directions for Use.
5. Patel R, Karp C, Yoo S, Amescua G, Galor A. Cataract surgery after refractive surgery. Int Ophthalmol Clin. 2016;56(2):171–182. doi: 10.1097/IIO.0000000000000106.
6. Naderi K, Gormley J, O’Brart D. Cataract surgery and dry eye disease: a review. Eur J Ophthalmol. 2020;30(5):840–855. doi: 10.1177/1120672120929958.
7. Gibbons A, Ali TK, Warren DP, Donalson KE. Causes and correction of dissatisfaction after implantation of presbyopia-correcting intraocular lenses. Clinical Ophthalmology. 2016:10 1965–1970.
IMPORTANT PRODUCT INFORMATION - AcrySof® IQ PanOptix® and Vivity Family of IOLs
CAUTION: Federal (USA) law restricts this device to the sale by or on the order of a physician.
INDICATIONS
The AcrySof® IQ PanOptix® Trifocal IOL, AcrySof® IQ PanOptix® Toric, AcrySof® IQ Vivity™ Extended Vision IOL and AcrySof® IQ Vivity™ Toric IOLs are indicated for visual correction of aphakia in adult patients following cataract surgery. In addition, the AcrySof Toric IOLs are indicated to correct pre-existing corneal astigmatism at the time of cataract surgery. The AcrySof® IQ PanOptix® lens mitigates the effects of presbyopia by providing improved intermediate and near visual acuity, while maintaining comparable distance visual acuity with a reduced need for eyeglasses, compared to a monofocal IOL. The AcrySof® IQ Vivity™ lens mitigates the effects of presbyopia by providing an extended depth of focus. Compared to an aspheric monofocal IOL, the lens provides improved intermediate and near visual acuity, while maintaining comparable distance visual acuity. All of these IOLs are intended for placement in the capsular bag
WARNINGS/PRECAUTIONS: Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting a lens in a patient with any of the conditions described in the Directions for Use labeling. Physicians should target emmetropia, and ensure that IOL centration is achieved.
For the PanOptix® Toric and Vivity™ IOLs, the lens should not be implanted if the posterior capsule is ruptured, if the zonules are damaged, or if a primary posterior capsulotomy is planned. Rotation can reduce astigmatic correction; if necessary lens repositioning should occur as early as possible prior to lens encapsulation.
For the AcrySof® IQ PanOptix® IOL, some visual effects may be expected due to the superposition of focused and unfocused multiple images. These may include some perceptions of halos or starbursts, as well as other visual symptoms. As with other multifocal IOLs, there is a possibility that visual symptoms may be significant enough that the patient will request explant of the multifocal IOL. A reduction in contrast sensitivity as compared to a monofocal IOL may be experienced by some patients and may be more prevalent in low lighting conditions. Therefore, patients implanted with multifocal IOLs should exercise caution when driving at night or in poor visibility conditions. Patients should be advised that unexpected outcomes could lead to continued spectacle dependence or the need for secondary surgical intervention (e.g., intraocular lens replacement or repositioning). As with other multifocal IOLs, patients may need glasses when reading small print or looking at small objects. Posterior capsule opacification (PCO), may significantly affect the vision of patients with multifocal IOLs sooner in its progression than patients with monofocal IOLs.
For the AcrySof® IQ Vivity™ IOL, most patients implanted with the Vivity™ IOL are likely to experience significant loss of contrast sensitivity as compared to a monofocal IOL. Therefore, it is essential that prospective patients be fully informed of this risk before giving their consent for implantation of the AcrySof® IQ Vivity™ IOL. In addition, patients should be warned that they will need to exercise caution when engaging in activities that require good vision in dimly lit environments, such as driving at night or in poor visibility conditions, especially in the presence of oncoming traffic. It is possible to experience very bothersome visual disturbances, significant enough that the patient could request explant of the IOL. In the AcrySof® IQ Vivity™ IOL clinical study, 1% to 2% of AcrySof® IQ Vivity™ IOL patients reported very bothersome starbursts, halos, blurred vision, or dark area visual disturbances; however, no explants were reported.
Prior to surgery, physicians should provide prospective patients with a copy of the Patient Information Brochure available from Alcon informing them of possible risks and benefits associated with these IOLs.
ATTENTION: Reference the Directions for Use labeling for each IOL for a complete listing of indications, warnings and precautions.
© 2023 Alcon Inc. 03/23 US-VIV-2300006






